Sleep And Cirrhosis
High Prevalence of Sleep Disturbance in Cirrhosis
HEPATOLOGY, February 1998, p. 339-345, Vol. 27, No. 2
Original Articles
Juan Córdoba1, Juan Cabrera1, Louis Lataif1, Plamen Penev2, Phyllis Zee2, and Andrés T. Blei1
From Northwestern University, and the Departments of 1 Medicine and 2Neurology, the Sleep Laboratory at Northwestern Memorial Hospital and Lakeside VA Medical Center, Chicago, IL
ABSTRACT
Sleep disturbance is a classic sign of hepatic encephalopathy. However, there are limited data regarding its prevalence in cirrhotic patients without overt hepatic encephalopathy. We assessed the characteristics of sleep in cirrhosis using a sleep questionnaire (n = 44) and actigraphy (n = 20). The results were compared with those of subjects with chronic renal failure and those of healthy controls. Presence of subclinical hepatic encephalopathy, chronotypology profile, and individual’s affective state were also analyzed. The questionnaire indicated an elevated number of cirrhotic patients (47.7%) and patients with chronic renal failure (38.6%) who complained of unsatisfactory sleep compared with healthy controls (4.5%, P < .01). Actigraphy corroborated the deterioration of sleep parameters in cirrhotic patients with unsatisfactory sleep. The sleep disturbance in cirrhosis was not associated with clinical parameters nor with cognitive impairment. Cirrhotic subjects and patients with chronic renal failure with unsatisfactory sleep showed higher scores for depression and anxiety, raising the possibility that the effects of chronic disease may underlie the pathogenesis of sleep disturbance. However, in contrast to chronic renal failure, unsatisfactory sleep in cirrhosis was associated with delayed bedtime, delayed wake-up time, and evening chronotypology. In conclusion, a sleep disturbance is frequent in cirrhotic patients without hepatic encephalopathy and may be related to abnormalities of the circadian timekeeping system. (HEPATOLOGY 1998;27:339-345.)
INTRODUCTION
A disturbance of sleep is recognized as one of the early signs of hepatic encephalopathy.1 However, there are limited data regarding its prevalence in patients with cirrhosis without signs of overt hepatic encephalopathy. Results from a quality of life questionnaire indicated that disturbance in sleep was significantly higher in nonalcoholic cirrhotic patients compared with subjects with another chronic illness, such as Crohn’s disease.2 In this survey and subsequent data,3up to 35% of cirrhotic individuals had difficulties in the area of sleep and rest.
The mechanisms responsible for these findings are poorly understood. One possibility is that abnormalities in circadian function may underlie its pathogenesis. The sleep-wake cycle is one of the functions regulated by the circadian clock, the suprachiasmatic nucleus of the anterior hypothalamus,4 which has efferent connections that influence a large array of biological functions including the secretion of melatonin from the pineal gland. Previous studies from our laboratory have shown that rats subjected to a portacaval anastomosis experience an alteration in the rhythm of circadian locomotor activity as well as the rhythm of pineal melatonin content.5 In patients with cirrhosis, the diurnal plasma melatonin profile showed a significant delay in the onset of plasma melatonin increase and in its peak nocturnal level.6 This displacement of the melatonin profile could be a reflection of an alteration in the phase of the circadian clock.7
We undertook a prospective study to assess the prevalence of sleep disturbance in a group of cirrhotic patients without overt encephalopathy using a sleep questionnaire, a robust tool for gauging quality of sleep.8 The results were compared with those of subjects with chronic renal failure (CRF) and healthy controls. Potential factors that could be related with a sleep disorder in these patients, such as liver function, the presence of subclinical hepatic encephalopathy, the chronotypology profile, and the individual’s affective state, were also analyzed.
We also evaluated the characteristics of sleep in an additional group of cirrhotic patients by means of actigraphy. The actigraph is based on a miniaturized acceleration sensor that translates physical motion to a numeric representation. It can be attached to the wrist and allows continuous monitoring of motor activity. The analysis of the periods of rest and activity provides a reasonable estimation of the time spent asleep and awake. 9,10 Although less precise than polysomnography, actigraphy has the advantage of monitoring subjects while they perform their customary social activities, avoiding the constraints of the sleep laboratory. Assessment of activity over several 24-hour periods helps to identify circadian-related factors that may contribute to the development of sleep disturbance. 10,11
PATIENTS AND METHODS
Sleep Survey
Patients With Cirrhosis. We studied a consecutive series of 44 cirrhotic patients without clinical evidence of hepatic encephalopathy. All the patients with the diagnosis of cirrhosis seen at the outpatient Liver Clinic of Northwestern University between December 1995 and February 1996 were invited to participate in the study. Twenty-two men and 22 women, whose mean age was 51 ± 2 years (range, 37-69 years) were included.
The diagnosis of cirrhosis was based on a compatible clinical history, radiological studies, and liver biopsy when available (59%). All of the patients showed evidence of portal hypertension. At the time of evaluation, most of them were Child-Pugh class A patients (73%); the rest were class B. The etiology of cirrhosis was Hepatitis C in 20 subjects, alcoholism in 12, Hepatitis B in 3, primary biliary cirrhosis in 4 (none with bilirubin >2 mg%, and diverse causes in 5. All patients were abstinent for at least a 6-month period (average, 17 ± 3 months); abstinence was ascertained by questioning patients and relatives. Patients with active alcoholism, history of drug abuse, those affected by neurological or psychiatric diseases, and individuals receiving psychotropic medications, such as benzodiazepines, were excluded. No patient was on treatment with interferon.
Twelve patients were being treated with diuretics because of ascites. Furosemide and spironolactone, usually at a low dose (furosemide 40 mg/d, spironolactone 100 mg/d) were the most commonly used drugs. Diuretics were prescribed at a single morning dose to avoid nocturia; at the time of inclusion into the study, ascites was mild or absent. Fifteen patients had suffered a previous variceal bleeding. For this reason, three patients were submitted to surgical portal-systemic derivations and two had a transjugular intrahepatic portosystemic shunt placed. Thirteen patients were on propranolol therapy as part of the treatment of portal hypertension. The dose (average, 20 mg twice daily; range, 20-160 mg/d) was titrated to cardiac frequency. Two patients were following a program of sclerosis or banding of esophageal varices; time between last session and inclusion into the study was over 3 months in all cases. None had suffered a previous episode of spontaneous portal-systemic encephalopathy or had chronic changes in mental state requiring therapy; three patients had a transient and minor change in mental state (stages I-II) during a previous episode of variceal bleeding. Half of the cirrhotic patients were listed for liver transplantation at our institution.
Controls. Gender- and age-matched disease controls were selected from patients with chronic renal failure (CRF) included in a waiting list for renal transplantation at our institution. The list included 250 individuals; once potential matches were identified by age and sex, subjects received a letter explaining the nature of the study, with a follow-up telephone call. Individuals were excluded for similar reasons as cirrhotic patients. All CRF subjects were being treated with either chronic hemodialysis (n = 30) or peritoneal dialysis (n = 14). Mean age of the patients with CRF was 50 ± 2 years, with half the group being of either sex.
A normal control group was selected from healthy subjects on no medications who responded to advertisement notices, most of them employees from our institution. Age of the normal controls was 50 ± 2 years, with a similar gender distribution.
Subclinical Encephalopathy. Cirrhotic patients were submitted to neuropsychological assessment to detect the presence of subclinical cognitive abnormalities. For this reason they were asked to perform a short battery of tests designed to detect impairment in the domains of attention and motor performance, which we have shown to be the most frequently impaired domains in patients with cirrhosis and normal consciousness.12 The battery consisted of the Trailmaking test (parts A and B), the Gordon Continuous Performance test, and the Grooved Pegboard test, all given by the same operator. In the Trailmaking test the subject connects numbers (part A) and alternating numbers and letters (part B) with maximal celerity. In the Gordon test, a series of numbers are shown on the front display, and the subject is required to respond after a certain pair of numbers appears. Correct responses (maximum = 30) and reaction time are recorded. The distractibility task differs from the vigilance task in the level of attention demand required. The Grooved Pegboard test consists of a board with parallel rows of holes into which grooved pegs are placed as quickly as possible.
Sleep Evaluation. All subjects were assessed at 9 AM with a structured interview of 30-minute duration. A sleep questionnaire used at the Sleep Clinic at Northwestern Memorial Hospital was completed. It consists of 55 questions that evaluate subjective appraisal of sleep quality (sleep satisfaction) and parameters of sleep such as total sleeping time, sleep latency (time to fall asleep), and number of awakenings during the night. Questions also referred to the characteristics of daytime functioning, such as excessive sleepiness and naps during daytime. In addition, the questionnaire included screening for common sleep disorders.
Chronotypology. The influence of chronotypology on sleep was explored with the Horne and Ostberg’s questionnaire.13 This questionnaire consists of 13 questions scored from 1 to 4-5. Statements make reference to morningness or eveningness feelings and preferences in the performance of diverse tasks. High values (maximum = 55) categorized individuals as “larks” and low values (minimum = 13) as “owls.” Customary bedtime and wake-up time were used as additional phase markers.
Depression and Anxiety. The Beck Depression Inventory (BDI), a self-report scale for the measurement of depression,14 was administered after the sleep questionnaire to all groups. Patients responded to 21 symptoms/attitude categories by rating each symptom item with a score ranging from 0 (absent) to 3 (severe). The scale is scored by summing the 21 responses (range, 0-63). This test is considered a screening test that may facilitate the recognition of depression by nonpsychiatric physicians. Scores >17 can be considered as evidence of moderate depression.15
The state of anxiety of the individuals was assessed by the S-anxiety questionnaire of the State-Trait Anxiety Inventory (STAI).16 Patients responded to 20 statements by rating each question with a score ranging from 1 (not at all) to 4 (very much so). The scale is scored by summing the 20 responses (range, 20-80).
Wrist Actigraphy
Subjects. An additional group of cirrhotic patients with similar characteristics to those included in the sleep survey was evaluated by means of wrist actigraphy. The study included 20 patients (male/female, 11:9; age, 52 ± 2 years) and 20 age- and sex-matched healthy controls (male/female, 11:9; age, 52 ± 2 years). Cirrhotic patients (10 Child-Pugh A, 10 Child-Pugh B) were randomly selected from the outpatient clinic at Northwestern Memorial Hospital. The diagnosis of cirrhosis was based on a liver biopsy or a compatible clinical history with evidence of portal-systemic shunting. Etiologies included 9 subjects with Hepatitis C, 7 with alcoholic liver disease, and 4 with other etiologies. No patients had imbibed alcohol in the last 6 months. Patients were included in the study if they were able to keep an independent and active lifestyle. Control subjects were required to have good health and to not take any medications. Individuals with unusual schedules (e.g., shift workers) were excluded. The clinical evaluation of all subjects included a subjective assessment of quality of sleep.
Actigraphy. The actigraph (Actillume; Ambulatory Monitoring Co., Ardsley, NY) was worn on the wrist for 5 days (Monday to Friday); weekends were avoided to favor stable routines. The equipment, slightly larger than the size of a watch, is battery operated, and continuously measures wrist movements. It contains as a sensor a piezoelectric device capable of detecting acceleration in all three axes of movement. The signal is sampled 20 times per second. A mean value, which averages the degree of activity that occurred over the selected time period, is stored. In our study an average was taken and stored every minute. The data stored in the device’s memory are downloaded to a compatible computer using a commercially available program (Action 3; Ambulatory Monitoring Co.) and can be viewed on screen or printed as hard copy. Data are expressed in artificial units. Manual adjustment is necessary to eliminate the periods in which the subject takes the equipment off for any reason (e.g., shower, swimming). In addition to activity data, the monitor has the ability to record light intensity and has a channel where specific events can be marked. The individual was instructed to press the button that registers specific events at bedtime and wake-up and to keep a log of main activities.
Sleep Parameters. The program that analyzes the acquired data can score activity/inactivity and derive from it periods of sleep or wakefulness using an algorithm (Fig. 1). The algorithm has been validated by comparing wrist actigraphy and polysomnographic recordings. 9,10,17 In our study, the following parameters were evaluated: 1) time in bed, duration of the period of time lying in bed for sleeping purposes, confirmed by event recordings and data from the light channel; 2) sleep efficiency, ratio between sleeping time and time in bed expressed as a percentage; 3) number of awakenings, number of episodes of awakening during time in bed; 4) wake after sleep onset, time awake after the start of sleeping time. The values were calculated averaging 4 nights (Monday to Friday).
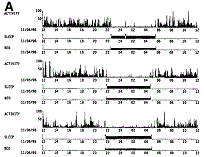
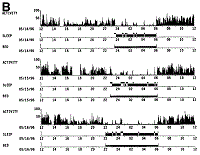
| Fig. 1. Examples of actigraphy of (A) a healthy control and (B) a cirrhotic patient with unsatisfactory sleep over three consecutive days. Each day is shown in one panel, with activity data in the upper trace. Based on the analysis of the activity data it is possible to estimate wake (upper horizontal line of the middle trace) and sleep (lower horizontal line of the middle trace) periods during the time spent in bed (horizontal line of the lower trace). |
Statistical Analysis
Results are expressed as means ± SEM. Statistical significance in contingency tables was evaluated using ![]() 2 and Fisher’s Exact test. Unpaired Student’s t test, one-way ANOVA, and Mann-Whitney rank sum test were used for comparisons of continuous variables. For significant differences (P < .05), multiple comparisons were performed using Dunn’s test. Repeated-measures ANOVA was used for analysis of the distribution of motor activity among the group; because of an unbalanced number of individuals per group and the elevated number of measures the Greenhouse-Geisser correction was applied. Performance of this study was approved by the Institutional Review Board of Northwestern University, and all patients gave written consent for participation.
2 and Fisher’s Exact test. Unpaired Student’s t test, one-way ANOVA, and Mann-Whitney rank sum test were used for comparisons of continuous variables. For significant differences (P < .05), multiple comparisons were performed using Dunn’s test. Repeated-measures ANOVA was used for analysis of the distribution of motor activity among the group; because of an unbalanced number of individuals per group and the elevated number of measures the Greenhouse-Geisser correction was applied. Performance of this study was approved by the Institutional Review Board of Northwestern University, and all patients gave written consent for participation.
RESULTS
Sleep Questionnaire. The characteristics of sleep are presented in table 1. Cirrhotic and CRF patients showed a significantly higher prevalence of sleep disturbance than the healthy control group. Almost half of the cirrhotic patients (47.7%) and more than one third of CRF patients (38.6%) complained of unsatisfactory sleep, whereas this complaint was infrequent in the healthy subjects (4.5%). Unsatisfactory sleep was present for 5 or more years in only three cirrhotic patients and in 2 CRF patients. The comparison of parameters of nighttime sleep between the different groups showed a significantly higher proportion of subjects referring short sleeping time (<6 h/night), difficulties falling asleep (sleep latency >30 minutes) and more frequent nocturnal awakenings in the cirrhotic and CRF groups. In addition, daytime functioning of these patients was affected by higher episodes of undesired sleepiness and more prolonged napping time.
| table 1. Characteristics of Sleep Among Groups |
In cirrhosis and CRF, unsatisfactory subjective sleep quality was associated with worsening objective parameters of nighttime sleep and daytime functioning (table1). In the healthy control group, the small number of individuals with unsatisfactory sleep precludes meaningful associations.
Clinical Characteristics. No difference was observed between cirrhotic patients with satisfactory (n = 21) and unsatisfactory sleep (n = 23) with regard to age, gender, Child-Pugh score, history of alcoholism or treatment with diuretics or propranolol (table 2). Etiology of cirrhosis, previous development of ascites, and previous variceal bleeding episodes were also similar in both groups. Laboratory data, including bilirubin, albumin, and prothrombin time, did not differ between both groups.
| View This table | table 2. Clinical Profile of Patients With Cirrhosis |
There were no differences between CRF patients with and without sleep disturbance in relation to age (51 ± 2 v. 49 ± 2 years), gender (male/female, 7:10 v. 15:12), dialysis type (hemodialysis/peritoneal dialysis, 10:7 v. 20:7), and time in dialysis (34 ± 5 v. 38 ± 7 months).
Subclinical Encephalopathy. In 24 of the 44 cirrhotic patients (54%), two or more psychometric tests showed an abnormal value (>2 SD above the mean of normative data). No differences could be observed in the characteristics of the cognitive profile between those patients with satisfactory and unsatisfactory sleep (table 3).
| table 3. Psychometric Tests in Cirrhosis |
Chronotypology. In the analysis of phase parameters as the reported bedtime or wake-up time and the morningness/eveningness score, cirrhotic patients with sleep disturbance showed a significant delay of the nocturnal period of rest and a morningness/eveningness score significantly higher than that for cirrhotic patients with normal sleep. In contrast, no differences were found in both of these phase parameters between CRF with and without sleep disturbance (Fig. 2).
| Fig. 2. Bedtime and wake-up time of patients with cirrhosis, chronic renal failure (grouped by sleep complaints), and healthy controls are plotted against the values of the Horne-Ostberg questionnaire (chronotypology profile). Results expressed as means ± SEM. *Cirrhotic patients with unsatisfactory sleep show a delayed bedtime, a delayed wake-up time, and a lower score (evening type) in this questionnaire compared with their satisfactory sleep counterparts (P < .05). |
Anxiety and Depression. Because of a clerical error, 10 cirrhotic patients did not receive the STAI anxiety questionnaire and the Beck Depression Inventory (BDI). Overall, the proportion of cirrhotic patients with evidence of moderate depression (BDI > 17) was 26% (9 of 34); in patients with CRF this proportion was 20% (9 of 44). None of the healthy controls showed a BDI > 17.
Comparative analysis of cirrhotic patients showed that STAI anxiety state score and the BDI score were significantly higher in patients with unsatisfactory sleep (STAI, 48 ± 3; BDI, 20 ± 2) than in those with satisfactory sleep (STAI, 30 ± 2, P< .0001; BDI, 5 ± 1, P < .0001). Similarly, higher levels of anxiety and depression were also found among CRF subjects with unsatisfactory sleep (STAI, 40 ± 3; BDI, 16 ± 2) compared with their satisfactory sleep counterparts (STAI, 31 ± 2, P= .02; BDI, 8 ± 1, P = .007). The mean scores for STAI (31 ± 1) and BDI (3 ± 1) in the healthy control group did not differ from those in patients with satisfactory sleep. An analysis of the results of these tests excluding somatic items18 did not modify the results.
Wrist Actigraphy
Sleep Characteristics. Seven of the 20 cirrhotic patients (35%) assessed with wrist actigraphy complained of unsatisfactory sleep. As in the first part of the study, cirrhotic patients with unsatisfactory sleep did not show distinct clinical characteristics when compared with the group with satisfactory sleep. Analysis of sleep parameters obtained with actigraphy indicated a fragmented nocturnal sleep and deterioration in sleep parameters, in accordance with their subjective assessment (table 4).
| table 4. Quality of Sleep Assessed by Wrist Actigraphy |
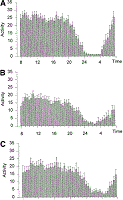
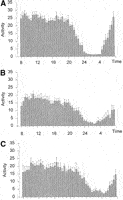
Activity. In cirrhosis patients, the level of motor activity during 24 hours was decreased (12.7 ± 0.9 v. 18.2 ± 1.1 in controls; P < .001). The decrease of motor activity in cirrhosis was unevenly distributed during the 24-hour day. Indeed, probably as a reflection of fragmented sleep, nocturnal activity was increased in cirrhosis (2.0 ± 0.3 v. 1.1 ± 0.1 in controls; P < .01). This resulted in a dampening of the circadian rhythm of motor activity as expressed by the relation night/day activity (cirrhosis, 11.6% ± 1.2% v. controls, 4.9% ± 0.5%, P < .0001). Differences in the distribution of motor activity were more obvious in cirrhotic patients with unsatisfactory sleep (Fig. 3), who in accordance to the chronotypology profile detected in the sleep survey showed a shift of activity toward later hours of the day.
| Fig. 3. Activity data in (A) healthy controls (n = 20), (B) cirrhotic patients with satisfactory sleep (n = 13), and (C) cirrhotic patients with unsatisfactory sleep (n = 7). The graphs show the average activity of 5 days plotted in intervals of 30 minutes (bars) and as best-fitted curve (line). Results are expressed as means ± SEM. Analysis of the data using repeated measures ANOVA with Greenhouse-Heisser correction showed a significant effect of group (P < .0001), time of the day (P < .0001), and interaction between group and time (P =.002). |
DISCUSSION
The present study shows that patients with cirrhosis without evidence of hepatic encephalopathy and who were evaluated while performing their daily routines have abnormalities in the quality of sleep. Nearly one half of patients attending a Liver Clinic complained of unsatisfactory sleep, a frequency slightly greater than that previously reported with quality of life questionnaires, 2,3 instruments that are not focused on the assessment of sleep. These results were corroborated by the objective parameters provided by actigraphy in a different patient sample, indicating that sleep complaints are not because of misperception.19 The prevalence of sleep complaints was similar to that of patients with chronic renal failure, a chronic disease commonly associated with sleep disturbance. 20,21Analysis of sleep questionnaires in both groups of patients showed a nocturnal sleep characterized by reduced sleeping time, prolonged conciliation time, and frequent awakenings. These results raise the possibility that our findings of disturbed sleep may be nonspecific, reflecting the impact of chronic disease on daily functions. Results from our university-based clinic may also reflect a more severely affected population.
Nonetheless, in the majority of patients the development of sleep disturbance had a duration of less than 5 years, supporting a causal relationship with the development of chronic liver disease. This causal relationship is also supported by the substantial improvement in the area of sleep that has been observed after liver transplantation.22 We could not identify clinical characteristics that distinguished cirrhotic patients with unsatisfactory sleep from their satisfactory sleep counterparts. Most of the patients were well-compensated Child-Pugh class A cirrhotic patients that were otherwise asymptomatic and kept their usual occupations. Complications of portal hypertension were equally distributed and did not result in a different pattern of medications, such as propranolol, a drug that has been related to sleep disturbance.23 Inclusion in a waiting list for liver transplantation or previous variceal bleeding, factors that may have resulted in a higher level of anxiety, were also similar in both groups. In this group of alcohol-abstinent patients, the etiology of cirrhosis was not a discriminant factor, in accordance with recent observations on noncognitive abnormalities that were seen in all types of cirrhosis.24
Hepatic encephalopathy is associated with the development of circadian abnormalities. Rats after portacaval anastomosis, which represent a model of subclinical encephalopathy,25 show disruption of circadian rhythms.5 Thus, it is plausible to hypothesize that sleep disturbance in cirrhosis may be a manifestation of minor forms of encephalopathy. The prevalence of cognitive impairment detected in our patients with a short neuropsychological battery that detects attention and motor deficits12 is in accordance with previous reports.26However, there were no differences in the results of neuropsychological tests between individuals with or without sleep complaints, suggesting that the mechanisms that cause sleep disturbance are independent from those responsible for the cognitive abnormalities detected by such tests.
We have previously postulated6 that in cirrhosis, alterations of the function of the suprachiasmatic nucleus![]() the hypothalamic biological clock
the hypothalamic biological clock![]() may result in an array of circadian abnormalities. 5,6,27-30 Desynchronization between the social and the internal rhythm mediates the transient insomnia that appears with jet lag or shift work.31 Likewise, sleep abnormalities may be secondary to malfunctioning circadian timekeeping systems,32 such as sleep disturbance, that develop with aging.33 Analysis of sleep patterns in cirrhotic patients with sleep disturbance indicated that these subjects had a delayed bedtime, delayed wake-up time, and preference for evening activities as compared with those with normal sleep. In contrast, no differences in these parameters were seen in patients with CRF with and without abnormal sleep. Moreover, in cirrhosis with unsatisfactory sleep, actigraphy showed a shift of activity toward later hours. A propensity for evening activity could reflect an alteration of circadian function, 34,35 in which there is a shift toward later hours as a result of an altered output from the circadian clock or its afferent/efferent connections. In a previous study, we observed in cirrhosis a displacement toward later hours in the 24-hour profile of plasma melatonin,6which has levels that reflect the output from the circadian clock. Accordingly, it can be hypothesized that the sleep disturbance in cirrhosis may be related to desynchronization of the circadian timekeeping system.7 The inversion of sleep pattern described in patients with overt encephalopathy1 could be an extreme of this displacement. Several mechanisms may be involved in the development of circadian abnormalities in cirrhosis, including the effect of gut-derived toxins on the brain36 and decreased sensorial inputs that entrain the circadian clock37such as insufficient light exposure, social isolation, or low levels of activity and retinohypothalamic38 and endocrine (e.g., melatonin) abnormalities.
may result in an array of circadian abnormalities. 5,6,27-30 Desynchronization between the social and the internal rhythm mediates the transient insomnia that appears with jet lag or shift work.31 Likewise, sleep abnormalities may be secondary to malfunctioning circadian timekeeping systems,32 such as sleep disturbance, that develop with aging.33 Analysis of sleep patterns in cirrhotic patients with sleep disturbance indicated that these subjects had a delayed bedtime, delayed wake-up time, and preference for evening activities as compared with those with normal sleep. In contrast, no differences in these parameters were seen in patients with CRF with and without abnormal sleep. Moreover, in cirrhosis with unsatisfactory sleep, actigraphy showed a shift of activity toward later hours. A propensity for evening activity could reflect an alteration of circadian function, 34,35 in which there is a shift toward later hours as a result of an altered output from the circadian clock or its afferent/efferent connections. In a previous study, we observed in cirrhosis a displacement toward later hours in the 24-hour profile of plasma melatonin,6which has levels that reflect the output from the circadian clock. Accordingly, it can be hypothesized that the sleep disturbance in cirrhosis may be related to desynchronization of the circadian timekeeping system.7 The inversion of sleep pattern described in patients with overt encephalopathy1 could be an extreme of this displacement. Several mechanisms may be involved in the development of circadian abnormalities in cirrhosis, including the effect of gut-derived toxins on the brain36 and decreased sensorial inputs that entrain the circadian clock37such as insufficient light exposure, social isolation, or low levels of activity and retinohypothalamic38 and endocrine (e.g., melatonin) abnormalities.
A relationship between sleep abnormalities and affective disorders has been frequently observed.39 Individuals with primary insomnia (psychophysiological insomnia) are usually characterized as mildly depressed, anxious, and somatically focused.40 We found significantly higher scores for BDI and STAI in patients with cirrhosis and CRF who complained of unsatisfactory sleep, which could arise as a consequence of insomnia. Alternatively, some of our patients may have shown an unrecognized affective disorder. Nevertheless, an important observation in our study is the different chronotypology profile between cirrhosis and CRF with sleep abnormalities in spite of similar scores on the BDI and the STAI questionnaires, which points at possible circadian abnormalities as the source for a sleep disturbance. Furthermore, the chronotypology profile in cirrhotic patients with unsatisfactory sleep differed from the circadian abnormalities that are usually associated with affective disorders, which are characterized by a phase advancement instead of a phase delay.7
In conclusion, the current study highlights the importance of sleep disturbance in patients with compensated cirrhosis. In addition, our data suggest a possible relationship of sleep complaints and alteration of circadian timekeeping systems. Recognition of sleep disturbance in cirrhosis and understanding its underlying pathophysiological mechanisms may result in approaches that translate into a better quality of life for such patients. From this perspective, actigraphy may be a useful tool for assessing sleep and monitoring the effect of specific therapies in subjects with cirrhosis.
Footnotes
Abbreviations: CRF, chronic renal failure; BDI, Beck Depression Inventory; STAI, State-Trait Anxiety Inventory.
Supported by a Merit Review from the Veterans Administration Research Service and the Blowitz-Ridgeway Foundation. Dr. Juan Cabrera was supported by a grant from the Spanish Government (FIS 95/5375) and by Fundación Universitaria de Las Palmas. Dr. Juan Córdoba was supported by a grant from Generalitat de Catalunya (CIRIT)
Received June 3, 1997; accepted September 26, 1997.
Address reprint requests to: Andrés T. Blei, M.D., Lakeside Veterans Affairs Medical Center, Department of Medicine, Room 111 E, 333 E. Huron St., Chicago, IL 60611. Fax: (312) 908-0036.
Copyright © 1998 by the American Association for the Study of Liver Diseases.