HEPATOLOGY, September 1998, p. 677-682, Vol. 28, No. 3
Original Articles
Effects of Propranolol on the Hepatic Hemodynamic Response to Physical Exercise in Patients With Cirrhosis
Juan-Carlos Bandi,
From the Hepatic Hemodynamic Laboratory, Liver Unit, Department of Medicine, IDIBAPS, Hospital Clinic i Provincial, University of Barcelona, Spain.
Abstract
Physical exercise increases portal pressure (hepatic venous pressure gradient [HVPG]) in patients with cirrhosis. It is unknown if this deleterious effect is associated with changes in gastroesophageal collateral blood flow and if these can be prevented by propranolol administration. The aim of this study was to characterize the effects of propranolol on the splanchnic hemodynamic response to exercise in patients with cirrhosis. Twenty-three patients with cirrhosis and portal hypertension had hemodynamic measurements in baseline conditions, and during moderate cycling exercise (40 W) under double-blind propranolol or placebo administration. In patients receiving placebo, HVPG significantly increased during exercise (from 16.7 ± 0.9 to 19.0 ± 1.0 mm Hg; P < .01), hepatic blood flow (HBF) decreased (-18% ± 4%; P < .01), while azygos blood flow (AzBF) was unchanged (4% ± 12%; ns). In patients receiving propranolol, portal pressure did not increase during exercise, but decreased from 16.3 ± 1.0 to 12.9 ± 1.1 mm Hg (P < .01). The lack of increase in HVPG in response to exercise in patients receiving propranolol may be related to a more pronounced decrease in HBF, as compared with patients receiving placebo, and to a blunted increase in cardiac output (CO). Moderate physical exercise adversely influences the hepatic hemodynamics in patients with cirrhosis, causing a significant increase in portal pressure. This is effectively prevented by propranolol pretreatment. (HEPATOLOGY 1998;28:677-682.)
Introduction
A previous study from our laboratory showed that physical exercise significantly increases portal pressure, as estimated from measurements of the hepatic venous pressure gradient (HVPG) in cirrhotic patients.1 The increase in HVPG during physical exercise occurred despite a fall in hepatic blood flow (HBF), suggesting that an increase in hepatic vascular resistance could be the responsible mechanism.1,2 However, whether the increase in portal pressure caused by physical exercise is associated with changes in gastroesophageal collateral blood flow has not been investigated. If present, an increase in the collateral blood flow could aggravate the deleterious effects of increasing portal pressure, worsening the risk of variceal bleeding in patients with portal hypertension.3,4
The present study was aimed at characterizing the effects of a moderate physical exercise on hepatic hemodynamics and collateral blood flow in patients with cirrhosis and portal hypertension. In addition, we examined whether propranolol therapy may prevent the worsening of portal hypertension caused by physical exercise.
Patients and Methods
Patients. The study was performed in 23 patients with cirrhosis who were referred to the Hepatic Hemodynamic Laboratory at the Liver Unit for evaluation of portal hypertension. Only compensated cirrhotic patients, physically fit and able to run a normal, active life, were asked to participate. All patients presented clinical evidence of portal hypertension: all had esophageal varices at endoscopy, and 4 of them had mild ascites. Twenty-one patients were male, and 2 were female; the mean age was 50.4 ± 5.8 years (mean ± SEM). The etiology of cirrhosis was alcoholic in 11 patients and associated with chronic Hepatitis C infection in the remaining 12. The mean Child-Pugh score was 6.1 ± 0.6 points. No patient had evidence of intrinsic pulmonary or cardiac disease, as confirmed by a normal chest x-ray film and electrocardiogram. No patients were receiving vasoactive drugs. Additional clinical data of these patients are reported in table 1. The protocol was approved by the Ethical Research Committee of the Hospital Clinic of Barcelona in 1995. Informed written consent to participate in the study was obtained from all patients.
| View This table | table 1. Clinical Data of the Patients Studied |
Procedures.
At 9:00 AM, after fasting overnight and under local anesthesia, two catheter introducers (USCI International, Galway, Ireland) were placed into the right jugular vein by the Seldinger technique. One of them was used to advance a balloon catheter (Medi Tech, Cooper Scientific Corp., Watertown, MA) into the main right hepatic vein for repeated measurements of wedged (occluded) and free hepatic venous pressures.5 The other was used, in 11 patients, to advance a Swan-Ganz catheter (Edwards Laboratory, Los Angeles, CA) into the pulmonary artery for measurement of cardiopulmonary pressures and, in 12 patients, to advance a 7F coronary sinus continuous thermaldilution catheter (Webster Laboratories, Baldwin Park, CA) into the azygos vein for measurement of azygos blood flow (AzBF).6 Intravascular pressures were measured using highly sensitive pressure transducers (Hewlett-Packard, model 1280 C, Andover, MA), calibrated before each measurement. Portal pressure was estimated by the HVPG, the difference between wedged and free hepatic venous pressures. Measurements were performed at least by duplicate in each period of the study, and permanent tracings were obtained on a multichannel recorder (Hewlett-Packard, 7754 B). Heart rate (HR), mean arterial pressure (MAP), and arterial saturation of oxygen were measured noninvasively and recorded at 2-minute intervals throughout the study (Cardioswiss CM-8, Schiller, Switzerland). HBF was measured using a continuous infusion of indocyanine green (Serb, Paris, France), prepared in a solution containing 2% human serum albumin, infused intravenously at a constant rate of 0.2 mg · min-1. After an equilibration period of at least 40 minutes, three sets of simultaneous samples of peripheral and hepatic venous blood were obtained for the measurement of HBF following previously reported methods.7 Vascular resistance across the vascular bed (dyn · s · cm-5) was calculated as the ratio of the pressure gradient (mm Hg) to blood flow (L · min-1) × 80. The hepatic sinusoidal vascular resistance was estimated as HVPG/HBF × 80; systemic vascular resistance was estimated as [MAP – RAP]/CO × 80, in which RAP is the right atrial pressure, and CO is the cardiac output; and pulmonary vascular resistance (PVR) was estimated as [PAP – PCP]/CO × 80, in which PAP is the pulmonary artery pressure, and PCP is the pulmonary capillary wedged pressure.
In addition, a peripheral venous blood sample was drawn to measure norepinephrine levels, as previously described.8 Norepinephrine was measured by a radioenzymatic assay (Upjohn Diagnostics, Kalamazoo, MI) according to the Peuler and Johnson method. Normal values of plasma norepinephrine in our laboratory were 224 ± 110 pg · mL-1. These measurements were repeated in each period of the study (see below).
Study Design.
After the catheterization procedure described above, baseline measurements of systemic and splanchnic hemodynamics and plasma norepinephrine levels were performed. Then, the patients were randomized to receive in double-blind conditions an intravenous infusion of propranolol or placebo. A loading dose of 0.15 mg · kg-1 was infused over 15 minutes in a total volume of 20 mL of saline and followed by a constant infusion of 0.2 mg · h-1. Measurements were repeated after 20 minutes. Then, the patients began to exercise on a cycloergometer (Ergometry System 380, Siemens-Elema, Schönander, Sweden) placed on the examination table while maintaining the infusion of propranolol or placebo. The exercise was moderate and consisted of a constant workload of 40 W. According to our previous work in cirrhotic patients with similar liver function,1 this exercise represents approximately 30% of their peak workload. After reaching a steady-state exercise (not before minute 3), the hemodynamic measurements (HVPG, AzBF, or CO, HBF, and plasma norepinephrine) were repeated. The duration of the measurements required that the patients cycled for a total period of 8 to 12 minutes. Patients were instructed to stop the exercise if they experienced dizziness, chest pain, or symptoms other than discomfort. Measurements were performed with the patients lying on the examination table .
Hemodynamic data and blood samples were obtained and analyzed under double-blind conditions.
Statistical Analysis.
The results are reported as means ± SEM. One-way ANOVA with the Schaffe F test for repeated measurements was used in the statistical analysis of the results. Differences in all parameters between propranolol and placebo groups were assessed using the unpaired t test. Statistical significance was established atP < .05.
Results
Baseline Data.
There were no significant differences between the clinical characteristics and baseline hemodynamic parameters of the patients who received placebo or propranolol (table 1). All patients had severe portal hypertension, manifested by the presence of esophageal varices and by a marked increase in the HVPG and AzBF (table 2). Portal hypertension was accompanied by a hyperdynamic circulation, with high CO and low peripheral vascular resistance (table 3). These findings are similar to those observed in other series of portal hypertensive cirrhotic patients previously studied in our laboratory.1,9
| table 2. Splanchnic Hemodynamics in Baseline Conditions, After Placebo or Propranolol Administration, During Exercise and 30 Minutes After Recovery Period |
| table 3. Systemic Hemodynamics in Baseline Conditions, After Placebo or Propranolol Administration, During Exercise and 30 Minutes After Recovery Period |
Effects of Placebo or Propranolol Infusion on Hepatic and Systemic Hemodynamics in Resting Conditions (Before Exercise).
There were no significant changes in pre-exercise hepatic and systemic hemodynamics following placebo administration. These parameters varied very little (below 5%) (Tables 2 and 3). On the contrary, propranolol administration caused significant reductions in heart rate (-20.7% ± 1.3%), CO (-25.0% ± 2.1%), HVPG (-14.4% ± 1.4%), HBF (-27.7% ± 4.5%), and a pronounced reduction in AzBF (-42.2% ± 7.7%) (all changes P < .01) (Tables 2 and 3).
Norepinephrine levels were not modified after placebo (from 321 ± 63 to 308 ± 52 pg · mL-1; -3% ± 7%; ns) or propranolol (from 327 ± 67 to 347 ± 62 pg · mL-1; 6% ± 11%; ns) administration.
Systemic Hemodynamics During Exercise.
In patients receiving placebo, physical exercise significantly increased HR, MAP, and CO, and significantly decreased systemic vascular resistance (table 3). However, propranolol pretreatment blunted the increase in CO caused by exercise (Fig. 1). In patients receiving placebo, stroke volume increased significantly during exercise, while in patients receiving propranolol, there were no significant changes (table 3). In both groups, physical exercise caused a significant increase in mean pulmonary artery pressure and in pulmonary capillary wedged pressure, without changes in pulmonary vascular resistance (table 3).
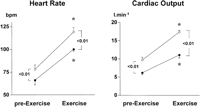 |
Fig. 1. Effects of moderate physical exercise on HR and CO in patients receiving propranolol ( |
When values during exercise were compared with baseline values, before propranolol or placebo infusion, the different behavior of both groups is further evidenced. Thus, the changes in HR and CO in the placebo group (HR: 53% ± 6% and CO: 81% ± 23%) were much higher than in the propranolol group (HR: 17% ± 4% and CO: 35% ± 4%; P < .05).
Norepinephrine levels increased markedly during exercise, from 308 ± 52 to 770 ± 135 pg · mL-1 in the placebo group (P < .01) and from 347 ± 62 to 1,193 ± 239 pg · mL-1 in the propranolol group (P < .001).
Splanchnic Hemodynamics During Exercise.
During exercise, patients receiving placebo showed a significant increase in HVPG (14% ± 5%; P < .01) (table 2). The increase in HVPG was observed in all patients studied, and was unrelated to baseline HVPG or other hemodynamic parameters. HBF decreased significantly (-18% ± 4%; P < .05) (table 2 As a consequence of the fall in HBF together with the increase in HVPG, the estimated hepatic sinusoidal vascular resistance increased markedly (48% ± 16%; P < .01) (table 2). In patients receiving placebo, AzBF was not changed during exercise (4% ± 12%; ns), suggesting that physical exercise did not modify gastroesophageal collateral blood flow.
On the contrary, in patients receiving propranolol, HVPG did not increase during exercise, but decreased significantly (-23% ± 4%; P < .01) (Fig. 2). HBF, which was significantly decreased by propranolol in resting conditions, further decreased during exercise (-26% ± 5%; P < .01). This decline was significantly greater than that observed in the placebo group (Fig. 3). AzBF was significantly decreased by propranolol before exercise (table 2) and remained steady during exercise, at values significantly lower than in patients receiving placebo (Fig. 3). The lack of increase in HVPG in response to exercise in patients receiving propranolol may be related to the more pronounced decrease in HBF as compared with patients receiving placebo (Fig. 3), and to the blunted increase in CO (Fig. 1).
| Fig. 2. Effects of moderate physical exercise on HVPG in patients receiving propranolol ( |
| Fig. 3. Effects of moderate physical exercise on AzBF and HBF in patients receiving propranolol ( |
Recovery Period.
Hemodynamic parameters were measured after a 30-minute recovery period, while maintaining the propranolol or placebo infusion. As shown in Tables 2 and3, upon recovery, all values had returned to pre-exercise values.
The protocol was well tolerated, and no complications related to the procedures were observed.
Discussion
Variceal bleeding is the most common and lethal complication of portal hypertension in patients with cirrhosis.10,11 A number of studies have shown that there is a direct relationship between the increase in portal pressure and the formation of varices,12,13 the risk of variceal bleeding14 and the mortality from variceal hemorrhage,15 all of these events being more common when HVPG increases during the follow-up, while decreasing when HVPG is reduced either spontaneously,14 because of alcoholic abstinence,16-18 or as the result of long-term pharmacological treatment with nonselective ![]() -blockers.14,19
-blockers.14,19
On the other hand, several recent studies have shown that the increased HVPG observed in portal hypertensive cirrhotic patients is not stable , but is significantly modified by several physiological stimuli. Thus, HVPG is significantly increased after a meal,20 which has been suggested to play a key role in promoting a progressive dilatation of the varices and bleeding, as well as in aggravating the bleeding episode and promoting early rebleeding.21 More recently, we described a daily variation of HVPG (following a circadian rhythm) in patients with cirrhosis.22 HVPG decreased significantly during the day and increased at night. These changes are matched by similar modifications in portal blood flow, as assessed by duplex-Doppler ultrasonography.23 The fact that these daily changes in HVPG and portal blood flow are paralleled by a similar daily variation of the time of occurrence of variceal bleeding does suggest that these physiological changes in HVPG are indeed of clinical significance.23 In that regard, it is of interest to note that propranolol therapy has been shown to attenuate or prevent the changes in HVPG and portal blood flow associated with meals,24,25 as well as its circadian variation.26
We have recently described how physical exercise significantly increases HVPG in patients with cirrhosis,1 thus adding another factor to the list of circumstances that may modify the splanchnic hemodynamics in cirrhosis. However, the possible effects of physical exercise on gastroesophageal collateral blood flow (which includes blood flow through the esophageal varices) was not studied in our previous investigation.1 More important, whether or not propranolol therapy may prevent the deleterious hemodynamic effects associated with physical exercise remained an open question.
The present study was designed to answer these questions. To make the results of the study comparable with the circumstances that the patients face in real life, the exercise load selected for this study was moderate (40 W), and roughly equivalent to that associated with daily activities such as washing dishes, performing housework, or walking at 4.5 mph,1,27 and were maintained for very short periods (8 to 12 minutes).
The results of this study confirm our previous report demonstrating a significant increase in HVPG during moderate physical exercise.1 The study further showed that this increase in portal pressure was not associated with a further increase in gastroesophageal collateral blood flow, as assessed by the measurement of AzBF. The lack of changes in AzBF contrasts with the decrease in HBF observed during exercise, suggesting a redistribution of the splanchnic blood flow to the collaterals, probably as a result of a less marked increase in the resistance to the collateral blood flow than to HBF during exercise. The maintenance of a high collateral blood flow together with the increase in HVPG does suggest that varices are subject to a marked stress during exercise, which is likely to result in a pronounced increase in variceal wall tension, facilitating the progressive dilatation of varices and augmenting the risk of variceal bleeding.3,28 These changes occur very rapidly during exercise and are of short duration, having totally disappeared after a 30-minute recovery period.
Probably the most important finding of the current study was the demonstration that propranolol administration totally prevented the increase in HVPG during exercise. Actually, the HVPG decreased during exercise in the patients receiving propranolol. This beneficial effect of propranolol during exercise occurred while maintaining the significant reduction in gastroesophageal collateral blood flow caused by ![]() -blockade. Thus, during exercise, patients treated with propranolol maintained a significantly (and markedly) lower HVPG and AzBF than patients receiving placebo, indicating that propranolol therapy affords adequate protection from the detrimental hemodynamic effects caused by moderate exercise, and therefore is likely to annulate the hypothetical clinical risks associated with such an increase in HVPG.
-blockade. Thus, during exercise, patients treated with propranolol maintained a significantly (and markedly) lower HVPG and AzBF than patients receiving placebo, indicating that propranolol therapy affords adequate protection from the detrimental hemodynamic effects caused by moderate exercise, and therefore is likely to annulate the hypothetical clinical risks associated with such an increase in HVPG.
The mechanisms by which HVPG does not increase, but decreases, during exercise in patients receiving propranolol is probably related to the circulatory effects of ![]() -blockers. Thus, patients treated with propranolol had a significantly less pronounced increase in HR in response to exercise, which was associated with a marked attenuation of the increase in cardiac index. Actually, the stroke volume did not increase at all in these patients. In addition, the splanchnic blood flow, as reflected by measuring the HBF, decreased more during exercise in patients receiving propranolol than in patients receiving placebo. Other effects of exercise on the systemic and pulmonary hemodynamics were similar to those reported in previous studies from our laboratory,1,29 as were the effects of propranolol pretreatment.
-blockers. Thus, patients treated with propranolol had a significantly less pronounced increase in HR in response to exercise, which was associated with a marked attenuation of the increase in cardiac index. Actually, the stroke volume did not increase at all in these patients. In addition, the splanchnic blood flow, as reflected by measuring the HBF, decreased more during exercise in patients receiving propranolol than in patients receiving placebo. Other effects of exercise on the systemic and pulmonary hemodynamics were similar to those reported in previous studies from our laboratory,1,29 as were the effects of propranolol pretreatment.
In summary, the results of the present study strongly suggest that propranolol therapy adequately protects from the deleterious effects of a moderate physical exercise on portal hemodynamics in cirrhosis. This may represent one of the mechanisms by which propranolol is effective in preventing bleeding and rebleeding from varices. However, our findings should not be taken as an invitation to advise physical exercise in portal hypertensive cirrhotic patients receiving propranolol. Indeed, although preventing an increase in HVPG and maintaining a reduced AzBF, propranolol pretreatment caused a more pronounced reduction in liver perfusion during exercise than that observed in patients receiving placebo, which may have adverse consequences in patients with an already impaired liver function.
Footnotes
Acknowledgement: The authors thank Ms. Diana Bird for her secretarial support, and Angeles Baringo, Laura Rocabert, and Rosa Saez for experttechnical assistance.
Abbreviations:
HVPG, hepatic venous pressure gradient; HBF, hepatic blood flow; AzBF, azygos blood flow; HR, heart rate; MAP, mean arterial pressure; CO, cardiac output.
Supported in part by grants from the Dirección General de Investigación Científica y Tecnológica (DGICYT PB 94-1562), and Fondo de Investigaciones Sanitarias (FIS 1309-97)
Received July 15, 1997; accepted April 30, 1998.
Address reprint requests to: Jaime Bosch, M.D., Hepatic Hemodynamic Laboratory, Liver Unit, Department of Medicine, Hospital Clinic, IDIBAPS, University of Barcelona, C/, Villarroel 170, 08036 Barcelona, Spain. Fax: 34-93-4515522.
References
- García-Pagán JC, Santos C, Barberá JA, Luca A, Roca J, Rodriguez-Roisin R, Bosch J, et al. Physical exercise increases portal pressure in patients with cirrhosis and portal hypertension. Gastroenterology 1996; 111: 1300-1306.
- Bosch J, García-Pagán JC, Feu F, Rodés J. Portal hypertension. Clinical pathobiology. In: Arias IM, Boyer JL, Fausto N, Jakoby WB, Schachter DA, Shafritz DA, eds. The Liver: Biology and Pathobiology. New York: Raven, 1994:1343-1355.
- Polio J, Grozmann RJ. Hemodynamic factors involved in the development and rupture of esophageal varices: a pathophysiological approach to treatment. Semin Liver Dis 1986; 6: 318-331.
- D’Amico G, Pagliaro L, Bosch J. The treatment of portal hypertension. A meta-analytic review. HEPATOLOGY 1995; 22: 332-354.
- Bosch J, Mastai R, Kravetz D, Bruix J, Gaya J, Rigau J, Rodés J. Effects of propranolol on azygos blood flow and hepatic and systemic hemodynamics in cirrhosis. HEPATOLOGY 1984; 4: 1200-1205.
- Bosch J, Groszmann RJ. Measurement of the azygos venous blood flow by continuous thermodilution technique: an index of blood flow through gastroesophageal collaterals in cirrhosis. HEPATOLOGY 1984; 4: 424-429.
- Garcia-Pagan JC, Feu F, Luca A, Fernandez M, Pizcueta P, Bosch J, Rodes J. Nicardipine increases hepatic blood flow and the hepatic clearance of indocyanine green in patients with cirrhosis. J Hepatol 1994; 20: 792-796.
- García-Pagán JC, Navasa M, Rivera F, Bosch J, Rodés J. Lymphocyte
 -2-adrenoceptors and plasma catecholamines in patients with cirrhosis. Relationship with the hemodynamic response to propranolol. Gastroenterology 1992; 102: 2015-2023.
-2-adrenoceptors and plasma catecholamines in patients with cirrhosis. Relationship with the hemodynamic response to propranolol. Gastroenterology 1992; 102: 2015-2023. - Luca A, Cirera I, García-Pagán JC, Feu F, Pizcueta P, Bosch J, Rodés J. Hemodynamic effects of acute changes in intra-abdominal pressure in patients with cirrhosis. Gastroenterology 1993; 104: 222-227.
- Burroughs AK, Bosch J. Clinical manifestations and management of bleeding episodes in cirrhotics. In: McIntyre N, Benhamou JP, Bircher J, Rizzeto M, Rodés J, eds. Oxford Textbook of Clinical Hepatology. Oxford: Oxford University Press, 1991:408-425.
- Burroughs AK, McCormick PA. Natural history and prognosis of variceal bleeding. Baillieres Clin Gastroenterol 1992; 6: 437-450.
- Garcia-Tsao G, Grozmann RJ, Fisher R, Conn HO, Atterbury CE, Glickmann M. Portal pressure, presence of gastroesophageal varices and variceal bleeding. HEPATOLOGY 1985; 5: 419-424.
- Rigau J, Bosch J, Bordas JM, Navasa M, Mastai R, Kravetz D, Bruix J, et al. Endoscopic measurement of variceal pressure in cirrhosis: correlation with portal pressure and variceal hemorrhage. Gastroenterology 1989; 96: 873-880.
- Groszmann RJ, Bosch J, Grace N, Conn HO, García-Tsao G, Navasa M, Albert J, et al. Hemodynamic events in a prospective randomized trial of propranolol vs placebo in the prevention of the first variceal hemorrhage. HEPATOLOGY 1990; 99: 1401-1407.
- Vinel JP, Cassigneul J, Levade M, Voigt JJ, Pascal JP. Assessment of short-term prognosis after variceal bleeding in patients with alcoholic cirrhosis by early measurement of portohepatic gradient. HEPATOLOGY 1986; 6: 116-117.
- McCormick PA, Morgan MY, Phillips A, Yin TP, McIntyre N, Burroughs AK. The effects of alcohol use on rebleeding and mortality in patients with alcoholic cirrhosis following variceal haemorrhage. J Hepatol 1992; 14: 99-103.
- Lebrec D, DeFleury P, Rueff B, Nahum H, Benhamou JP. Portal hypertension, size of esophageal varices and risk of gastrointestinal bleeding in alcoholic cirrhosis. Gastroenterology 1980; 79: 1139-1144.
- Vorobioff J, Groszmann RJ, Picabea E, Gamen M, Villavicencio R, Bordato J, Morel I, et al. Prognostic value of hepatic venous pressure gradient measurements in alcoholic cirrhosis: a 10-year prospective study. Gastroenterology 1996; 111: 701-709.
- Feu F, Garcia-Pagan JC, Bosch J, Luca A, Teres J, Escorsell A, Rodes J. Relation between portal pressure response to pharmacotherapy and risk of recurrent variceal haemorrhage in patients with cirrhosis. Lancet 1995; 346: 1056-1059.
- McCormick PA, Dick R, Graffeo M, Wagstaff D, Madden A, McIntyre N, Burroughs AK. The effect of non-protein liquid meals on the hepatic venous pressure gradient in patients with cirrhosis. J Hepatol 1990; 11: 221-225.
- McCormick PA, Jenkins SA, McIntyre N, Burroughs AK. Why portal hypertensive varices bleed and bleed: a hypothesis. Gut 1995; 36: 100-103.
- Garcia-Pagan JC, Feu F, Castells A, Luca A, Hermida RC, Rivera F, Bosch J, et al. Circadian variations of portal pressure and variceal hemorrhage in patients with cirrhosis. HEPATOLOGY 1994; 19: 595-601.
- Alvarez D, Golombeck D, Lopez P, de las Heras M, Viola L, Sanchez S, Kolker M, et al. Diurnal fluctuations of portal and systemic hemodynamic parameters in patients with cirrhosis. HEPATOLOGY 1994; 20: 1198-1203.
- Sabba C, Ferraioli G, Buonamico P, Mahl T, Taylor KJ, Lerner E, Albano O, et al. A randomized study of propranolol on postprandial portal hyperemia in cirrhotic patients. Gastroenterology 1992; 102: 1009-1016.
- Alvarez D, Miguez C, Podesta A, Terg R, Sanchez-Malo A, Bandi JC, Sanchez S, et al. Postprandial vascular response in patients with cirrhosis. Short-term effects of propranolol administration. Dig Dis Sci 1994; 39: 1288-1293.
- Alvarez D, de las Heras M, Abecasis R, Terg R, Gerona S, Albornoz L, Galdame O, et al. Daily variation in portal blood flow and the effect of propranolol administration in a randomized study of patients with cirrhosis. HEPATOLOGY 1997; 25: 548-550.
- Jones NL, Makrides L, Hitchcock C, Chypchart T, McCartney N. Normal standards for an incremental progressive cycle ergometer test. Am Rev Resp Dis 1985; 131: 700-708.
- Escorsell A, Bordas JM, Feu F, García-Pagán JC, Ginès A, Bosch J, Rodés J. Endoscopic assessment of variceal volume and wall tension in cirrhotic patients. Effects of pharmacological therapy. Gastroenterology 1997; 113: 1640-1646.
- Agustí AGN, Roca J, Rodriguez-Roisin R, Mastai R, Wagner PD, Bosch J. Pulmonary hemodynamics and gas exchange during exercise in liver cirrhosis. Am Rev Respir Dis 1989; 139: 485-491.
0270-9139/98/2803-0012$3.00/0
Copyright © 1998 by the American Association
for the Study of Liver Diseases.




