Hepatitisc
Ann Rheum Dis 1998;57:701-702 ( December )
Leader
Mixed cryoglobulinaemia after hepatitis C virus: more and less ambiguity
Article
Since the discovery of the association of hepatitis C virus (HCV) with mixed cryoglobulinaemia, the use of this term is more ambiguous than ever on the one hand; on the other hand, there is less ambiguity as 80% of what previously was classified as essential mixed cryoglobulinaemia (EMC) is now known as secondary to HCV infection. The ambiguity is generated mainly by the heterogeneity of mixed cryoglobulins that were originally defined as containing IgG-anti-IgG (rheumatoid factor (RF)) immune complexes. Early studies focused on these RF containing mixed cryoglobulins; most of the recent studies, which are predominantly studies on patients with HCV infection, include cryoglobulins characterised for mixed isotypes of immunoglobulins but not for RF activity.
Meltzer, Franklin and colleagues1 first coined the term “mixed cryoglobulins” for the cryoglobulinaemia composed of IgG and IgM RF that accompanied the syndrome of palpable purpura, arthralgias, and weakness. Studies performed by Franklin’s group and by others implicated the cryoglobulin components in the immune complex mediated vascular and glomerular lesions that occur in many patients with this syndrome. The Meltzer-Franklin syndrome, as it is now called, was also called essential mixed cryoglobulinaemia to distinguish it from similar mixed cryoglobulinaemia that was known to occur secondary to a variety of infectious, autoimmune, and malignant diseases.
Shortly after Meltzer et al1 published their study, the great immunologist, Kunkel2 noted that cryoglobulins might be a sensitive and, by far, the simplest method for the detection of immune complex because certain complexes may be soluble at 37°C but precipitate on standing at 4°C. Mixed cryoglobulinaemia came to imply immune complex disease. This implication was, in fact, true for the Meltzer-Franklin syndrome because immune complexes, that is, IgG-IgM RF, were demonstrated in the mixed cryoglobulins, and components of the complex were demonstrated in the main lesions.
The same admonition that I made 18 years ago for “immune complexes” detected by the ubiquitous “immune complex assays” of that era3 must be re-stated here even more emphatically for mixed cryoglobulins. Their presence can be taken only as putative evidence for immune complexes. High molecular weight immune complexes that can be dissociated into antigen and antibody must be demonstrated to establish the presence of immune complexes in the mixed cryoglobulins. To conclude that immune complexes are involved in a disease process, the immune complexes must be demonstrated in the main lesions of that disease. This may seem to be an obvious criterion, but it was ignored in the flood of studies performed using the “immune complex assays” and has been ignored in recent studies on mixed cryoglobulinaemia. Studies required to delineate immune complexes and the immune complex disease process are notoriously difficult to perform, and thus they are avoided. Ambiguity, if not confusion, is the price of this approach.
The biochemical classification of cryoglobulins that is currently widely used was formulated by Brouet et al4 six years after the description of the Meltzer-Franklin syndrome. This study, which defined three types of cryoglobulins, was based on the characterisation of the cryoglobulins from 86 patients who had various diseases—most with manifestations of immune complex disease. Type I cryoglobulins consisted of a single isotype of monoclonal immunoglobulins. Type II cryoglobulins consisted of a mixture of immunoglobulin isotypes with a monoclonal component processing antibody activity toward polyclonal IgG. The monoclonal components were predominantly IgM. Type III cryoglobulins consisted of a mixture of polyclonal immunoglobulins of various isotypes; most of these were also complexes of polyclonal IgG and polyclonal RF that were mainly IgM. Because RF was a component of most of the type II and type III cryoglobulins, this study was essentially a correlation of IgG-IgM RF complexes, with the manifestations of immune complex diseases.
Recent studies include cryoglobulins characterised for isotypes of immunoglobulins and monoclonal components but not for RF activity. Only the prevalence of RF in the serum is usually given. The report in this issue by Lee et al5 is an author-acknowledged example of such a study. In the degenerate form of the Brouet classification used in this and similar studies, there is the tacit assumption that if RF is present in the serum it must be in the cryoglobulin. This assumption may be correct, but the authors did not indicate the patients who had RF in the serum and whether cryoglobulins were present in those patients. The end result is that various immunoglobulin isotypes and monoclonal components are detected in the cryoglobulins, but not even indirect evidence for immune complexes is provided so that the level of ambiguity generated is higher than in earlier studies in which at least IgG-IgM RF complexes were identified.
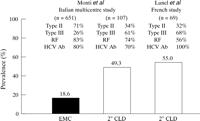
An example of confusion surrounding mixed cryoglobulins is the different views of cirrhosis among patients with mixed cryoglobulinaemia held by gastroenterologists and rheumatologists. Gastroenterologists believe the prevalence of cirrhosis is higher, whereas the rheumatologist believes it is low. The reason for this can be gleaned from the two studies compared in figure 1. In the Italian multicentre study,6 the prevalence of cirrhosis among patients with EMC was significantly lower than that in patients with cryoglobulinaemia secondary to chronic liver disease (CLD). The French study by Lunel, a gastroenterologist, and her colleagues7 of patients with mixed cryoglobulinaemia secondary to CLD found a prevalence of cirrhosis similar to that found for this group in the Italian study. The ratio of patients with type III to type II cryoglobulinaemia, which was the reverse of that found in EMC, was similar in the two CLD groups.
| Figure 1: Results of the Italian6 and French7studies are compared. The prevalences of cirrhosis in essential mixed cryoglobulinaemia (EMC) and in cryoglobulinaemia secondary to chronic liver disease (2° CLD) are shown. The prevalences of the type of cryoglobulinaemia (II or III) and rheumatoid factor (RF) and antibodies to hepatitis C virus antigens (HCV Ab) in the serum are shown for each group. Reprinted from Agnello V,8 with permission of the publisher. |
Thus, the different “views” result from a patient selection bias. Patients with EMC similar to those in the Italian multicentre study on presentation frequently have palpable purpura and other manifestations of systemic vasculitis and are more likely to be seen by rheumatologists and other specialists than by gastroenterologists. Patients with CLD have overt liver disease and are seen by the gastroenterologist. However, these two populations of patients may be associated with distinct types of mixed cryoglobulins. Unfortunately, the analysis of the cryoglobulins in the two studies in figure 1 was not sufficient to establish whether a difference was present beyond the distribution of the cryoglobulin types. In both of these studies, RF activity in the cryoglobulins was not determined.
In the Italian study and in my review8 of 1033 patients with EMC, palpable purpura was the most prominent clinical manifestation of mixed cryoglobulinaemia, confirming the original observation of Meltzer et al. Palpable purpura and other manifestations of cutaneous vasculitis seem to be the hallmark of a vasculitis mediated by IgG-IgM RF complexes in mixed cryoglobulins. Recently, HCV has been demonstrated in the cutaneous vasculitic lesions of patients with type II cryoglobulinaemia.9 Taken together with the earlier finding that HCV was selectively concentrated in type II cryoglobulins with IgG and IgM RF,10 this finding suggested that the cutaneous vasculitic lesions result from deposition of complexes of HCV, IgM RF, and IgG.
In most of the cases in the Italian multicentre studies and in our studies that use sensitive methods for the detection of monoclonal IgM RF (Agnello V, unpublished data), the cutaneous lesions are associated with type II cryoglobulinaemia, suggesting that monoclonal IgM RF may be essential for the development of the cutaneous vasculitic lesions. Immunofixation is routinely used for characterisation of cryoglobulins. More precise characterisation of the type III cryoglobulins using the more sensitive technique of immunoblotting11and the analysis of RF activity of the monoclonal IgM component may determine that type III cryoglobulins associated with cutaneous vasculitis also contain a monoclonal IgM RF. Hence, the precise characterisation of the IgM RF in these cryoglobulins may help define new clinicopathological correlations.
The precise characterisation of cryoglobulins and correlation with clinical disease are critically needed for mixed cryoglobulins that are mixtures of immunoglobulin isotypes without RF activity. These mixed cryoglobulins may represent immune complexes distinct from those containing RF and may be associated with extrahepatic manifestations of HCV infection that are not associated with RF containing mixed cryoglobulins, for example, membranous glomerulonephritis or lichen planus. On the other hand, the immunoglobulins in these cryoglobulins may represent flotsam from the ineffective immune response to HCV infection that is a feature of this disease.12 In either instance, precise characterisation and correlation with clinical disease manifestations of mixed cryoglobulins may help diminish the ambiguity surrounding mixed cryoglobulinaemia and perhaps help delineate the role of some of these mixed cryoglobulins in the extrahepatic manifestations of HCV infection.
VINCENT AGNELLO
Department of Laboratory Medicine, Lahey Clinic Medical Center, Burlington, Massachusetts, and Edith Nourse Rogers Memorial Veterans Affairs Hospital, Bedford, Massachusetts, USA
Correspondence to: Dr Agnello, Department of Laboratory Medicine, Lahey Clinic Medical Center, 41 Mall Road, Burlington, Massachusetts 01805, USA.
REFERENCES
© 1998 by Annals of the Rheumatic Diseases