Transarterial Embolization Versus Symptomatic Treatment in Patients With Advanced Hepatocellular Carcinoma: Results of a Randomized, Controlled Trial in a Single Institution
HEPATOLOGY, June 1998, p. 1578-1583, Vol. 27, No. 6
Original Articles
Jordi Bruix1, Josep M. Llovet1, Antoni Castells1, Xavier Montañá2, Concepció Brú2, Maria Del Carmen Ayuso2, Ramon Vilana2, and Joan Rodés1
From the 1 Liver Unit and 2 Radiology Departments, IDIBAPS,* Hospital Clínic i Provincial, University of Barcelona, Barcelona, Catalonia, Spain.
ABSTRACT
This randomized, controlled trial assessed the effect of transarterial embolization (TAE) (without associated chemotherapy) on the survival of patients with nonsurgical hepatocellular carcinoma (HCC). Eighty consecutive patients were randomized to treatment with embolization (Group A, n = 40), or to symptomatic treatment (Group B, n = 40), there being no differences between both groups regarding the degree of liver function impairment and tumor stage. Eighty-two percent of the patients presented a self-limited postembolization syndrome, without treatment-related mortality. Fifty-five percent of the treated cases exhibited a partial response, which resulted in a lower probability of tumor progression during follow-up (57% vs. 77% at 1 year; P < .005). However, after a median follow-up of 24 months (30 deaths in each group), there are no differences in survival (Group A: 49% and 13%; Group B: 50% and 27%, at 2 and 4 years, respectively; P = .72). The absence of differences was maintained even when dividing patients according to Child-Pugh’s grade, Okuda stage, or performance status test (PST). Furthermore, there were no differences in the probability of complications or in the need of hospital admissions. In conclusion, TAE has a marked antitumoral effect associated to a slower growth of the tumor, but it does not improve the survival of patients with nonsurgical HCC. (HEPATOLOGY 1998;27:1578-1583.)
INTRODUCTION
Transarterial embolization (TAE), alone or combined with chemotherapy (the so-called chemoembolization), remains a controversial treatment approach for patients with hepatocellular carcinoma (HCC).1,2 Hepatic artery obstruction induces an extensive tumor necrosis, and this provided the rationale for its wide use in patients with HCC. However, two recent controlled trials in France using chemoembolization have failed to disclose a significant benefit in survival when comparing treated patients with untreated cases.3,4 Thus, it could be that the antitumoral effect of arterial obstruction is counteracted by deleterious side-effects caused by the obstruction by itself or caused by the toxicity of the antineoplastic agents injected at the time of the procedure. In fact, none of the available antineoplastic agents has been shown to bear a significant therapeutic efficacy,5 and for this reason, in our Liver Unit, we decided to perform embolization without chemotherapy. In a previous phase II trial, we confirmed the antitumoral effect of this option.6 Simultaneously, we estimated the potential benefit in survival by comparing the outcome of treated patients with the outcome that would be expected according to a mathematical model derived from a previous series of patients.7 The estimated improvement in survival was significant, but we realized that it was so moderate that it was needed to perform the present randomized, controlled trial to fully confirm the clinical usefulness of this treatment. Taking into account the palliative nature of TAE, we designed the study not only to assess the effects on survival, but also in other end-points such as tumor progression kinetics, maintenance of the physical condition, and occurrence of cancer-related complications.
PATIENTS AND METHODS
In our Liver Unit, patients with HCC are diagnosed, staged, and treated according to a previously published schedule.6,8,9 In brief, the diagnosis of the neoplasm is ensured by needle-biopsy and/or by increased ![]() -fetoprotein levels. The tumor stage is established by ultrasonography, dynamic computed tomography (CT), and angiography, and bone metastases are ruled out by scintigraphy. The degree of liver function impairment is estimated by means of routine biochemical parameters reflecting liver and renal function. Patients with solitary small HCC are first evaluated for surgical resection. If it is not feasible because of liver function impairment,10 they are considered for orthotopic liver transplantation. The criteria for rejecting a patient for transplantation are tumor size larger than 5 cm, presence of more than one tumor site or vascular invasion, age above 65 years, and presence of severe associated diseases. If surgery is contraindicated, the patients are considered for percutaneous ethanol injection.8 Patients with HCC > 4 cm and/or multinodular are considered for TAE. If this procedure is precluded because of portal vein thrombosis and/or extrahepatic spread and/or presence of contraindications to a peripheral artery catheterization, the patients are considered for other medical options, such as chemotherapy, immunotherapy, or hormonal treatment. Finally, patients with disease that is well advanced belonging to Okuda stage III11 or presenting a performance status test (PST)12 greater than 2 receive only symptomatic treatment.
-fetoprotein levels. The tumor stage is established by ultrasonography, dynamic computed tomography (CT), and angiography, and bone metastases are ruled out by scintigraphy. The degree of liver function impairment is estimated by means of routine biochemical parameters reflecting liver and renal function. Patients with solitary small HCC are first evaluated for surgical resection. If it is not feasible because of liver function impairment,10 they are considered for orthotopic liver transplantation. The criteria for rejecting a patient for transplantation are tumor size larger than 5 cm, presence of more than one tumor site or vascular invasion, age above 65 years, and presence of severe associated diseases. If surgery is contraindicated, the patients are considered for percutaneous ethanol injection.8 Patients with HCC > 4 cm and/or multinodular are considered for TAE. If this procedure is precluded because of portal vein thrombosis and/or extrahepatic spread and/or presence of contraindications to a peripheral artery catheterization, the patients are considered for other medical options, such as chemotherapy, immunotherapy, or hormonal treatment. Finally, patients with disease that is well advanced belonging to Okuda stage III11 or presenting a performance status test (PST)12 greater than 2 receive only symptomatic treatment.
Accordingly, the present study includes non-previously treated HCC patients not suitable for surgical resection, liver transplantation, or percutaneous ethanol injection. Exclusion criteria were age over 75 years, uncontrolled liver disease decompensation (gastrointestinal bleeding, encephalopathy, bacterial infection), presence of portal vein thrombosis, extrahepatic spread, or any contraindication for an arterial procedure such as impairment of clotting tests (platelet count < 50.000/mm3 or prothrombin activity < 50%), renal failure, or severe atheromatosis.
Patients fulfilling the above criteria were asked to give their written informed consent to enter the study, which was approved by the Investigation and Ethics Committee of the Hospital. To guarantee the identity between the two arms of the trial, the patients were stratified before randomization into eight categories according to tumor stage (uninodular vs. multinodular), Okuda’s stage (I vs. II), and baseline PST (0-1 vs. 2). Randomization was performed using a computer-generated allocation, dividing each category into two groups: Group A: patients receiving TAE; Group B: patients receiving only symptomatic treatment.
Transarterial Embolization. The procedure was performed after an overnight fast by the patients and following a common schedule. Hydration of the patients was performed through a central line. The femoral artery was catheterized under local anesthesia, and a catheter was guided into the hepatic artery under continuous fluoroscopic control. After identifying the tumoral nodules by angiography, the catheter was advanced to obtain the most selective occlusion of the feeding artery. At that level, a mixture of radiological contrast and small cubes (1 × 1 mm) of gelatin was injected until achieving absence of flow. In patients with unilobar disease, the distal embolization with gelatin was combined, if technically feasible, with the proximal placement of a steel coil, thus aiming to produce a complete occlusion of the vessel and to enhance the ischemic effect. No intra-arterial chemotherapy was given.
After hepatic artery occlusion, the patients were carefully observed in a conventional hospitalization room, and analgesics (pentazocine or meperidine) were administered if necessary. Oral intake was reinitiated as soon as possible according to the tolerance of the patients. After confirming the absence of clinical abnormalities, the patients were discharged and followed in the outpatient clinic.
Assessment of Response. The effects of TAE on the tumor were assessed by dynamic CT within the first month after treatment. The presence of nonenhanced tumoral areas reflects tissue necrosis, and according to the findings of this imaging technique, the response to treatment was defined according to the World Health Organization criteria13: complete response: no evidence of neoplastic disease; partial response: reduction in total tumor load of >50%; no change: reduction of <50% or increase of <25%; progressive disease: increase of >25%.
Follow-up. All the patients were controlled at least every 3 months in the outpatient clinic by means of clinical examination, biochemistry, and ultrasound. Dynamic CT was performed every 6 months. Bone metastases were ruled out by bone scintigraphy if clinically suspected. In addition to conventional clinical and biochemical parameters, recorded data included the classification of the patients according to the Child-Pugh’s score,14 the Okuda staging system, and the PST.
If a patient had responded initially to TAE and had new tumoral nodules seen on ultrasonography or CT, or if the initial lesions appeared to be revascularized, the possibility of performing a new TAE was considered by using the same criteria as for the first treatment.
Patients presenting liver decompensation (gastrointestinal bleeding, hepatic encephalopathy, ascites, bacterial infections) during follow-up received the same treatment as non-neoplastic liver disease patients. Pain was treated avoiding the use of nonsteroidal anti-inflammatory agents, which are known to induce renal failure in patients with decompensated liver disease.15 The follow-up was maintained until death.
Statistical Methods. The sample size calculation was performed according to the results reported in the previously published phase II trial.6 Expecting an overall survival of 20% at 2 years in the control group and aiming to achieve a 30% increase in the TAE-treated group, and requiring a level of statistical significance of 0.05 and a power of 0.80, we planned to include 40 patients in each group.
The baseline and follow-up characteristics/events of the patients are given as means ± SD. Comparison between groups was performed by using the Student’st test for quantitative variables with parametric distribution and the Mann-WhitneyU test for those with nonparametric distribution. Qualitative variables were compared by means of the ![]() 2 test, applying the Yates’ method when needed. Probability curves of overall and cancer-related survival, tumor progression, development of complications, and maintenance of the PST were calculated according to the Kaplan-Meier method, comparing the curves by the Mantel-Cox test. Predictive factors of survival and the treatment group were subsequently included in a stepwise-forward Cox regression analysis to avoid any confounding interaction between them. The follow-up was closed on December 31, 1996. All the calculations were performed by using the BMDP statistical package (Biomedical Computer Programs, University of California Press, Berkeley, CA).
2 test, applying the Yates’ method when needed. Probability curves of overall and cancer-related survival, tumor progression, development of complications, and maintenance of the PST were calculated according to the Kaplan-Meier method, comparing the curves by the Mantel-Cox test. Predictive factors of survival and the treatment group were subsequently included in a stepwise-forward Cox regression analysis to avoid any confounding interaction between them. The follow-up was closed on December 31, 1996. All the calculations were performed by using the BMDP statistical package (Biomedical Computer Programs, University of California Press, Berkeley, CA).
RESULTS
Between January 1992 and April 1994, we evaluated 345 HCC patients. Eighty patients fulfilled the criteria to enter the trial and were stratified and randomized into the two treatment arms. Forty patients were treated by TAE (Group A), and 40 received only conservative treatment (Group B). As reflected in table 1, both groups were identical with regard to age, sex, etiology of cirrhosis, presence of constitutional syndrome, degree of liver function impairment, and tumor stage, which was estimated by the number of tumor nodules (solitary versus multinodular/massive), their location (unilobar vs. multilobar), and increase in ![]() -fetoprotein concentration. All of the patients had cirrhosis, and there were no differences when classifying them according to the Child-Pugh’s score, to the Okuda’s stage, or to the baseline PST.
-fetoprotein concentration. All of the patients had cirrhosis, and there were no differences when classifying them according to the Child-Pugh’s score, to the Okuda’s stage, or to the baseline PST.
| View This table | table 1. Baseline Characteristics of the Patients |
Hepatic artery blood flow to the tumor was completely abolished in 38 patients. In the remaining 2 cases, a minor part of the vascular supply to the lesion could not be obstructed because of the presence of abnormal hepatic vascularization precluding complete TAE. In 18 of the 24 patients with unilobar disease, the procedure included the placement of a steel coil.
TAE was well tolerated. Thirty-three of the 40 patients (83%) developed a self-limited postembolization syndrome, consisting of fever, abdominal pain, and nausea. There were no treatment-related deaths.
Antitumoral Effect and Tumor Growth. None of the patients treated by TAE achieved a complete response. Partial response was obtained in 22 patients (55%), while no changes were achieved in 14 patients (35%). Finally, 4 patients exhibited tumor progression. TAE was repeated in 16 patients upon detection of disease progression after an initial favorable response, the mean number of treatments in these patients being 2.2.
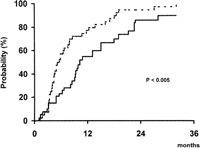
In addition to this marked antitumoral effect, patients treated by TAE showed a significantly lower probability of presenting tumor progression during follow-up than patients of the control group. As shown in Fig. 1, at 1 year of follow-up, 57% of Group A and 77% of Group B have developed disease progression (P < .005). By contrast, there were no differences between both groups with regards to the probability of developing portal vein thrombosis (30% vs. 19% at 2 years, respectively; P = .24) or extrahepatic spread (23% vs. 16% at 2 years, respectively; P = .92) during follow-up.
|
|
Fig. 1. Probability of presenting tumor progression during follow-up in patients treated by TAE (continuous line) and in patients of the control group (dotted line). |
Physical Condition and Development of Complications. The probability of maintaining the baseline PST, an index of the physical condition of the patient, was similar in the two groups (46% vs. 30% at 2 years; P = .08). Furthermore, there were no differences in the 2-year probability of developing complications (ascites [51% vs. 61%; P = .79], variceal bleeding [22% vs. 14%; P = .86], bacterial infection [6% vs. 32%; P = .19], hepatic encephalopathy [16% vs. 15%;P = .71], or in the need for hospital admission [23 vs. 24 patients; P = .85]) during follow-up.
Similarly, the probability of presenting abdominal pain (43% vs. 53% at 2 years, respectively; P = .53) and constitutional syndrome (31% vs. 50% at 2 years, respectively; P = .22) during follow-up was similar in the two groups.
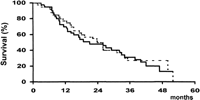
Survival. At the time of analysis, 30 patients from Group A and 30 from Group B had died; the causes of death are shown in table 2. After a similar mean follow-up of both groups (Group A: 23 ± 15 months; Group B: 24 ± 14 months), there were no differences in survival (Fig. 2), the 2- and 4-year rates being 49% and 13% vs. 50% and 27%, respectively (P = .72). Furthermore, the absence of differences subsided when patients were divided according to baseline Child-Pugh’s class, Okuda’s stage, or PST (table 3), and when only cancer-related deaths were considered (56% vs. 50% at 2 years, respectively; P = .86). Finally, TAE-treated patients who achieved a partial response after treatment had a similar survival than patients of the control group (58% vs. 50% at 2 years, respectively; P = .80).
| View This table | table 2. Causes of Death |
|
|
Fig. 2. Probability of survival in patients treated by TAE (continuous line) and in patients of the control group (dotted line). |
| table 3. Probability of Survival of the Patients According to Their Baseline Characteristics |
A preserved PST (P = .005) and a lower concentration of serum bilirubin (P = .05) were identified as variables associated with better survival. When these variables and the treatment group were included in the Cox regression analysis, TAE treatment did not enter the model.
DISCUSSION
Only a minor proportion of the patients currently diagnosed with HCC will be classified as having an early small HCC that may benefit from radical options, such as surgical resection, liver transplantation, or ethanol injection. Thus, the majority of the HCC patients will be considered for some of the available palliative approaches, there being no clear-cut evidence that any of them results in an improved outcome.1,5 Among these options, one of the most frequently applied is TAE, associated or not to the selective administration of chemotherapy, usually mixed with lipiodol (so-called “chemoembolization”). The tumor blood supply depends mainly on the hepatic artery, and thus, its obstruction results in extensive tumor necrosis caused by ischemia. The addition of chemotherapy aims to enhance the antitumoral action of ischemia, and the selective injection of a mixture of lipiodol and antineoplastic agent into the hepatic artery is expected to increase the drug delivery to the tumor cells, while simultaneously decreasing the risk of systemic side-effects. Some of these assumptions have not been properly substantiated,16-18 and it must be emphasized that the lack of effects of chemotherapy is not caused by a poor delivery of the drug, but rather by the expression of the multidrug resistance gene and/or an abnormal p53 function,19 a characteristic that will not be solved by any of these technical modifications. Thus, in patients treated by chemoembolization, the potential advantages of tumor mass reduction caused by ischemia may be counteracted by the deleterious side-effects induced locally and systemically by the administration of chemotherapy. In that regard, two recent controlled studies comparing chemoembolization (with adriamycin or cisplatinum) versus no treatment have shown that despite achieving a remarkable rate of responses to treatment, there is no improvement in survival.3,4
The absence of data supporting a benefit of combining chemotherapy with arterial embolization prompted us several years ago to avoid the use of antineoplastic agents and to base the treatment of those patients diagnosed at an intermediate evolutionary stage on the obstruction of the hepatic artery blood flow. In a previously published phase II trial, we confirmed that TAE was highly effective, because most of the patients exhibited a positive response to the treatment.6 In addition, we encountered that the survival of the patients treated by TAE was significantly better than the survival predicted by a mathematical model derived from a historical series. Because we considered that the observed difference in survival could be biased because of the nature of the comparison, we decided that we were fully justified in performing a controlled trial comparing TAE versus no treatment.
The present study has confirmed again that TAE is able to induce an extensive tumor necrosis in most of the patients. However, this has not resulted in an improvement of the survival of treated patients. Furthermore, the absence of differences has been maintained even when restricting the analysis to those patients presenting a partial response (tumor mass reduction > 50%) or to those exhibiting tumor necrosis of any extent. In addition, we also examined if there was a special subgroup of patients, as defined by liver function or by tumor stage, in whom TAE was able to improve survival, and the results were also negative. Finally, we analyzed the prognostic factors of these patients, and in the multivariate approach, we included treatment as a variable; it did not reach statistical significance.
It could be argued that our failure to identify the survival benefit of an otherwise evident response to treatment is the consequence of a methodological defect in the selection of cases, in the application of treatment, or in the follow-up. As reflected in our criteria to select patients to enter the trial, we have included patients with nonsurgical HCC who had not reached a terminal stage. This excludes patients suitable for surgical resection, liver transplantation, or ethanol injection who are expected to have early HCC, and eliminates those individuals in whom the extreme deterioration of their liver function and/or the advanced stage of the tumor imply a very poor prognosis that will be hardly modified by any therapeutic approach. To guarantee the identity between the two treatment arms, we stratified the patients according to Okuda stage and PST before randomization. Therefore, both groups were identical, there being almost no option for a bias caused by an unbalanced allocation of the patients. Regarding the technique used to perform the hepatic artery obstruction, it could be suggested that the placement of metallic coils to enhance the ischemic effect of the obstruction with gelfoam is not merited and that, in addition, it could prevent the re-embolization of the patients upon detection of tumor progression after an initial response. Furthermore, it could be argued that both gelfoam and the coils will provide a more proximal obstruction than agents expected to act distally, such as polyvinyl alcohol or gelfoam powder, and that this proximal obstruction could enhance the development of parasitic blood supply coming from phrenic or omental arteries, or aberrant collaterals precluding further embolization. However, gelfoam powder may induce ischemic bile duct necrosis,20 and polyvinyl alcohol has not been extensively used in patients with HCC; thus, before using it in randomized, controlled trial, detailed phase II studies should define its safety and efficacy. Nevertheless, it must be taken into account that our response rate (55%) to treatment is almost the same as those reported in studies using gelfoam, combined or not with chemotherapy (30%-70%), and that the survival reported in those studies supposedly following a more aggressive re-embolization schedule is almost the same as the one obtained in our study.4,21-27 Finally, there is no evidence showing that the repetition of the treatment is of any benefit; therefore, the usefulness of a more invasive retreatment policy, even at fixed periods of time, should be investigated within a prospective trial. When considering the follow-up as a potential source of bias, it must be noted that both groups were equally followed and the appearance of complications was managed according to the same criteria. In fact, a heterogeneous follow-up should be expected to favor treated patients and thus to induce a better survival in these patients.
In addition to survival, our study assessed potential differences in the progression of the neoplastic disease and in the appearance of cancer-related complications. Tumor progression during follow-up was observed in most of the cases, but its probability along time was significantly lower in treated patients. However, this was not paralleled by a difference in the appearance of cancer-related complications or in the causes of death: TAE did not affect the probability of presenting pain, and both groups exhibited a similar pattern in terms of deterioration of the PST. Furthermore, there were no differences in the timing and frequency of complications reflecting a deterioration of the liver function such as ascites, jaundice, variceal bleeding, or encephalopathy. It is probable that the lack of clinical translation of the slower tumor growth reflects that the conventional requirements to define tumor progression are not sensitive enough to properly register as tumor progression the growth of the tumor required to induce clinical symptoms. The mechanism by which an evident reduction in tumor mass is not paralleled by an improvement in survival, or at least in a reduction or delay in the appearance of complications, is not known. The obstruction of the hepatic artery may deteriorate the liver function of the patients, but this is a transient effect, and 1 week after treatment, the liver function is fully recovered.28 It could be speculated that the treatment by itself has both beneficial effects and detrimental consequences, and these last ones would counteract the advantages of tumor mass reduction. TAE may be associated to the appearance of mRNA for ![]() -fetoprotein in peripheral blood, a marker reflecting the presence of circulating malignant cells.29,30 Obviously, cells released after TAE will not homogeneously give place to new tumor sites, but this iatrogenic release of cells may increase the risk of presenting additional HCC nodules that would be registered as tumor progression and potentially be responsible for the deterioration of the patients in the absence of relevant growth of the initial nodule. Another mechanism could be related to the heterogeneity of the cells forming the tumor. Malignant hepatocytes are less sensitive to hipoxia than normal hepatocytes, and this reduced sensitivity is mediated through p53,31which is less frequently abnormal in well-differentiated HCC.32 Thus, it might be that TAE affects mainly well-differentiated cells, while leaving in place those that are poorly differentiated, and therefore may exhibit a more aggressive behavior. While in untreated patients, the growth of these more advanced clones may be partially impeded by the surrounding tumoral tissue, in treated patients, this defensive structure might be completely abolished, which would allow the progression of clones with a more malignant phenotype, which ultimately will counteract the benefits caused by the necrosis of well-differentiated cells.
-fetoprotein in peripheral blood, a marker reflecting the presence of circulating malignant cells.29,30 Obviously, cells released after TAE will not homogeneously give place to new tumor sites, but this iatrogenic release of cells may increase the risk of presenting additional HCC nodules that would be registered as tumor progression and potentially be responsible for the deterioration of the patients in the absence of relevant growth of the initial nodule. Another mechanism could be related to the heterogeneity of the cells forming the tumor. Malignant hepatocytes are less sensitive to hipoxia than normal hepatocytes, and this reduced sensitivity is mediated through p53,31which is less frequently abnormal in well-differentiated HCC.32 Thus, it might be that TAE affects mainly well-differentiated cells, while leaving in place those that are poorly differentiated, and therefore may exhibit a more aggressive behavior. While in untreated patients, the growth of these more advanced clones may be partially impeded by the surrounding tumoral tissue, in treated patients, this defensive structure might be completely abolished, which would allow the progression of clones with a more malignant phenotype, which ultimately will counteract the benefits caused by the necrosis of well-differentiated cells.
The negative results obtained with TAE and chemoembolization could suggest that these therapeutic approaches must be completely abandoned. However, it must be stressed that both options achieve a high rate of positive responses, and perhaps instead of giving them up, it would be more adequate to ideate new treatment associations and/or schedules through which the initial treatment response could be enlarged and potentially maintained for a longer period. If not having an impact on survival, the achievement of a more intense and extended antineoplastic effect could at least delay the appearance of cancer-related complications. The evaluation of these new options will have to be performed within randomized, controlled trials including a very large number of patients. It might be that the results of these studies appear negative again in the whole set of patients, but the development of such large studies may identify a special subgroup of individuals in whom the treatment may significantly modify their outcome.
Footnotes
Abbreviations: TAE, transarterial embolization; HCC, hepatocellular carcinoma; CT, computed tomography; PST, performance status test.
* IDIBAPS: Institut d’Investigacions Biomèdiques August Pi i Sunyer.
Supported by a research grant FIS 93/00544 from the Fondo de Investigaciones Sanitarias.
Presented in plenary session at the Annual Meeting of the American Association for the Study of the Liver Diseases, Chicago, IL, 1997
Received February 5, 1998; accepted March 24, 1998.
Address reprint requests to: Jordi Bruix, M.D., Liver Unit, Hospital Clínic i Provincial, Villarroel 170, 08036-Barcelona, Catalonia. Spain. Fax: 34-3-4515522.
Copyright © 1998 by the American Association for the Study of Liver Diseases.