Early Hepatocellular Carcinoma as an Entity With a High Rate of Surgical Cure
Hepatology, November 1998, p. 1241-1246, Vol. 28, No. 5
Tadatoshi Takayama1,5,
From the Departments of 1Surgery, 2Internal Medicine, and 3Diagnostic Radiology,2> National Cancer Center Hospital; the 4Pathology Division, National Cancer Center Research Institute; and the 5Second Department of Surgery,Faculty of Medicine,University of Tokyo, Tokyo, Japan.
Abstract
Early hepatocellular carcinoma (HCC) has been defined as a well-differentiated cancer containing Glisson’s triad, but it remains unknown whether this lesion is curable. We prospectively studied 70 patients (enrolled from 1,172 referrals between 1982 and 1991) who had a diagnosis of a single HCC 2 cm or less in diameter (Stage T1) and who underwent curative hepatectomy and long-term follow-up (range, 0.2 to 14.3 years). Patients were eligible for surgery if they had a tumor that met the diagnostic criteria for HCC and were in Child-Pugh class A (n = 59) or B (n = 11) status. Among the 70 patients, there was 1 operative death. Based on our typing system, the tumors were assigned as early HCC (n = 15), overt HCC (n = 52), and non-HCC tumor (n = 3). The rate of microscopic regional spread was lower in early HCCs than in overt HCCs (7% vs. 42%; P = .01). The early HCC group had a longer time to recurrence than did the overt HCC group (3.9 vs. 1.7 years; P < .001) and had no local recurrence. After a median follow-up of 6.3 years, both overall survival and recurrence-free survival in the early HCC group were significantly better than those in the overt HCC group (P = .01; P= .001). In these two groups, the 5-year rates of overall survival were 93% and 54% (P = .01), and those of recurrence-free survival were 47% and 16% (P= .05), respectively; a significant survival benefit persisted over a decade (57% vs. 21%; P = .05). The early HCC group was at a lower risk of recurrence (relative risk, 0.31; 95% CI, 0.15 to 0.65; P = .002) and death (relative risk, 0.26; 95% CI, 0.09 to 0.73; P = .01) than was the overt HCC group. Early HCC is a distinct clinical entity with a high rate of surgical cure, thereby justifying its definition. It can be a lesion that corresponds to “Stage 0” cancer in other organs. (HEPATOLOGY 1998;28:1241-1246.)
Introduction
Early hepatocellular carcinoma (HCC) is still defined on histopathological grounds. We have proposed that early HCC is a well-differentiated cancer with no substantial destruction of the preexisting hepatic framework.1 This lesion resembles in situ or microinvasive carcinoma, because it seldom vasoinvades or metastasizes,1 originates from a precursor,2 lacks known genetic alterations,3and represents an initial stage of hepatocarcinogenesis.4
Whether patients with early HCC are more likely to benefit from therapeutic intervention than are those with overt HCC remains to be clarified. Evidence of a better response to treatment is needed to justify definition of this new entity, because the term “early” cancer denotes a lesion that is potentially curable. However, clinical trials assessing outcome in patients with early HCC have been precluded by difficulty in detection and diagnosis; in previous studies, most of the lesions were secondary tumors discovered by chance in resected specimens from patients with overt HCC.1,4 Therefore, our nomenclature based only on pathology appears to have limitations in a clinical setting, and the term “well- or very well-differentiated HCC,” suggested by the International Working Party,5may serve as an alternative classification until the clinical implications of early HCC are more clearly determined.
Recent progress in imaging techniques has facilitated recognition of early HCC as a principal tumor in at-risk subjects who undergo regular medical check-ups for chronic viral hepatitis or cirrhosis.6,7 We thus conducted a prospective study of surgical treatment for Stage I HCCs to assess the therapeutic impact of hepatectomy on long-term outcome in patients who had had an early HCC.
Patients and Methods
Patients. From 1982 through 1991, we sought patients with early HCC among 1,172 patients referred with a preliminary diagnosis of liver tumor. A total of 596 patients were excluded on the basis of laboratory data and examinations of ultrasonography and computed tomography (CT): 485 were given a diagnosis of far-advanced (n = 214), secondary or other malignant (n = 152), and benign (n = 119) liver tumors; 70 had poor hepatic function (Child-Pugh class C); 32 had equivocal findings in the liver; and 9 refused surgery. The remaining 576 patients were judged as having surgically treatable HCCs. Among them, 80 patients with clinical evidence of a single HCC 2 cm or less in diameter, with no vascular invasion (T1), lymph node metastasis (N0), or distant metastasis (M0) (Stage I HCC, according to the UICC tumor-node-metastasis classification system)8 were eligible for this study.
Diagnosis and Treatment.
Of the 80 patients with Stage I tumors, 76 (95%) had received regular check-ups at referring hospitals for a median of 5.4 years; 72 (90%) were found to have a tumor by ultrasonography. In these patients, HCC was diagnosed on the basis of six specific imaging findings: 1) mosaic nodular appearance on ultrasonography; 2) a low-high-low density profile during dynamic CT9; 3) tumor staining on angiography; 4) hypoattenuation on transarterial portographic CT10; 5) hyperattenuation on hepatic arteriographic CT10; and 6) deposition of Lipiodol on follow-up unenhanced CT. Nonspecific features obtained by these methods were considered to indicate that the tumor was “detectable ,” but were not considered “diagnostic.” The diagnostic criteria for HCC required unequivocally specific evidence of at least two of the first three imaging findings. In cases failing to satisfy this requirement, other imaging method(s) (up to six) were then used until at least two techniques confirmed the specific finding. Serum ![]() -fetoprotein concentrations were measured in all patients at enrollment.
-fetoprotein concentrations were measured in all patients at enrollment.
The 80 patients (55 men and 25 women; median age, 57 years; range, 30 to 74 years) had chronic hepatitis or cirrhosis caused by hepatitis B virus (surface antigen-positive, n = 15; related antibody alone, n = 33), hepatitis C virus (n = 37, of 48 patients who survived beyond 1990 when the assay became available), or both (n = 2). Patients whose tumor met the diagnostic criteria for HCC were scheduled to undergo hepatectomy, while those whose tumor did not were observed, with informed written consent from all patients. Ultrasound-guided local resection was the routine procedure, and segmentectomy was performed in patients whose tumor was located deep or was adjacent to the intrahepatic major vessels.11 The surgery was defined as curative when all gross lesions were removed with a tumor-free margin.
Pathology.
The resected tumors were typed according to our gross classification system for small HCC1 into “overt” types (type-1, single nodular; type-2, single nodular with extranodular growth; type-3, contiguous multinodular), “early” type, and others, by three liver pathologists who were unaware of clinical details. Tumor-cell differentiation was graded as 1, 2, or 3 (when heterogenous, the predominant grade was assigned) according to Edmondson’s system,12 and capsular formation and microscopic regional spread (vascular invasion and metastasis) of tumor were assessed. Nontumor parenchyma of the specimens was also examined to identify precancerous lesions, such as adenomatous hyperplasia (dysplastic nodule) or dysplastic foci, defined by mild hypercellularity (below twofold as compared with the background parenchyma) and lack of atypia and invasion.2,5 The final diagnosis was consistent with the histological criteria recommended by the International Working Party.5
The definition for early HCC was as follows: macroscopically, the tumor was a distinctive nodule from the surrounding lobules by its size or color, which did not substantially destroy the preexisting hepatic framework; microscopically, it had to contain Glisson’s triad (the portal tracts), and to show hypercellularity (over twofold) with minimal cellular or nuclear atypia (Edmondson’s grade-1), as well as definite structural atypia, as indicated by acinar formation, thin trabeculae, or remodeling of the cord architecture.1,4,13
Follow-up.
After surgery, all patients were screened by ultrasonography every 2 months and dynamic CT every 4 months (each interval was doubled after 5 years). When recurrence was suspected, angiography was added for diagnostic or therapeutic purposes. Recurrence was defined clinically as the appearance of a new lesion with radiological features typical of HCC, as confirmed by two imaging methods.14 Intrahepatic recurrence in relation to the initial tumor was classified s local, unilobar, or bilobar. Any tumor, regardless of the time to recurrence, arising in the same segment as the initial tumor (or within 2 cm from the surgical stump when performing segmentectomy) was considered a “local” recurrence. Patients found to have recurrence underwent a second hepatic resection (in those with a single tumor, preserved hepatic function, and the consent), percutaneous ethanol injection (in those with less than three tumors, each ![]() 3 cm), or trans-catheter arterial embolization. All patients were followed up for a minimum of 5 years (as of March 1997) or until death.
3 cm), or trans-catheter arterial embolization. All patients were followed up for a minimum of 5 years (as of March 1997) or until death.
Statistics. The characteristics of the primary tumor and postsurgical recurrence in patients with early HCC were compared with those in patients with overt HCC by means of the ![]() 2 test, Fisher’s Exact test, or Wilcoxon’s rank sum test, as appropriate. The cumulative probabilities of survival in the two patient groups were calculated by the Kaplan-Meier method and compared by the log rank test. The prognostic relevance of 16 variables with respect to recurrence and death was evaluated by univariate analysis with the log rank test and by multivariate analysis with the Cox proportional hazards model. Analyses were conducted with the SAS statistical package version 6.11 (SAS Institute Inc., Cary, NC). All tests were two-tailed, and P < .05 was taken to indicate statistical significance.
2 test, Fisher’s Exact test, or Wilcoxon’s rank sum test, as appropriate. The cumulative probabilities of survival in the two patient groups were calculated by the Kaplan-Meier method and compared by the log rank test. The prognostic relevance of 16 variables with respect to recurrence and death was evaluated by univariate analysis with the log rank test and by multivariate analysis with the Cox proportional hazards model. Analyses were conducted with the SAS statistical package version 6.11 (SAS Institute Inc., Cary, NC). All tests were two-tailed, and P < .05 was taken to indicate statistical significance.
Results
Clinical Outcome. Among 1,172 referrals in a decade, 80 patients with a preliminary diagnosis of Stage I HCC were enrolled. All the 80 patients underwent ultrasonography, dynamic CT, and angiography, which had detection rates of 99%, 89%, and 69%, and diagnostic rates of 65%, 68%, and 65%, respectively (table 1). There were 70 patients whose tumor met the diagnostic criteria for HCC. The diagnosis was established with these three imaging methods in 45 patients; four methods were required in 18 patients, and five or six methods in 7 patients. The addition of portographic CT permitted diagnosis in 13 patients, arteriographic CT in 5, both CTs in 2, and Lipiodol-CT in 5. The other 10 patients whose tumor did not meet the criteria were followed, in 7 of whom the tumor transformed to HCC (n = 3) or HCC(s) developed at other sites (n = 4) after a median of 3.7 years (range, 2.4 to 6.4 years).
| View This table | table 1. Diagnosis of Stage I Liver Tumors in 80 Patients |
The 70 patients (Child-Pugh class A, n = 59; class B, n = 11) underwent local resection (n = 53) or segmentectomy (n = 17). Intraoperative ultrasonography revealed that the tumor stage had been underestimated in 7 patients (10%): 5 had two tumors, 1 had three tumors, and 1 had a tumor measuring 3.0 cm. All seven newly found tumors (size, ![]() 1.0 cm) were also resected, and the cases were reassigned to Stage II tumor. The blood loss was 766 ± 539 mL (mean ± SD), and 11 patients (16%) required transfusions. All surgeries were curative, with a tumor-free surgical margin (5 ± 4 mm). One patient died of liver failure 3 months after surgery (in-hospital mortality, 1.4%).
1.0 cm) were also resected, and the cases were reassigned to Stage II tumor. The blood loss was 766 ± 539 mL (mean ± SD), and 11 patients (16%) required transfusions. All surgeries were curative, with a tumor-free surgical margin (5 ± 4 mm). One patient died of liver failure 3 months after surgery (in-hospital mortality, 1.4%).
Pathological Outcome. Based on the typing system (table 2), the tumors were classified as early HCCs (n = 15), overt HCCs (n = 52), and other tumors (n = 3; 2 dysplastic nodules and 1 hemangioma, which were excluded from further analysis). Early HCCs were smaller than overt HCCs (14 ± 3 mm vs. 17 ± 4 mm;P = .02). All early HCCs contained Glisson’s triad (12 ± 5 per tumor) and were Edmondson’s grade-1 (well-differentiated). Forty-nine of the overt HCCs showed no evidence of the triad or grade-1 areas, but in the other 3, both features were seen at the lesion periphery. The incidence of regional cancer spread was significantly lower in early HCCs (7%) than in overt HCCs (42%) (P = .01). Dysplastic nodules or foci coexisted in 47% of early HCC specimens and in 35% of overt HCC specimens (P = .55).
| View This table | table 2. Pathological Characteristics of HCC in 67 Patients |
Recurrence and Survival.
Postsurgical median follow-up for 67 patients with HCC was 6.3 years (range, 0.2 to 14.3 years). A total of 59 patients (88%) had recurrences in the remnant liver (table 3). Early (within 3 years) recurrence was significantly less frequent in the early HCC group (8%) than in the overt HCC group (74%) (P < .001). The early HCC group showed a trend toward a lower rate of multiple recurrence and had no local recurrence. The median time to recurrence in the early HCC group was significantly longer than that in the overt HCC group (3.9 vs. 1.7 years; P < .001), but the median survival after recurrence was not different.
| View This table | table 3. Postsurgical Recurrence of HCC in 59 Patients |
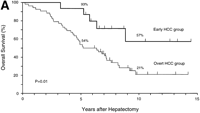
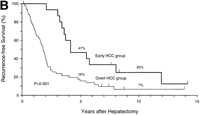
During follow-up, 42 patients (63%) died of recurrent HCC (n = 40), liver failure (n = 1), or sigmoid colon cancer (n = 1).As shown in Fig. 1, the rates of both overall survival and recurrence-freesurvival were significantly higher in the early HCC group thanin the overt HCC group (P = .01 for overall survival; P = .001 for recurrence-free survival). The 5-year rates of overall survivalwere 93% and 54% (P = .01), and those of recurrence-free survivalwere 47% and 16% (P = .05) in the two groups, respectively. Significantdifferences in overall survival persisted throughout the follow-upperiod (at 10 years and later, 57% vs. 21%; P = .05). There were8 long-term (over 10 years) survivors: 4 patients in the earlyHCC group (27%) and 4 in the overt HCC group (8%) (P = .07).
|
|
Fig. 1. Kaplan-Meier estimates of survival after hepatectomy in 67 patients with HCC, according to the tumor typing. (A) Overall survival and (B) recurrence-free survival in the early HCC group (n = 15) and the overt HCC group (n = 52). The 7 patients who finally proved to have Stage II HCC and the 1 patient who died of liver failure after 3 months (all belonged to the overt HCC group) were included. Tick marks indicate surviving patients (A) or those with no recurrence (B) and their duration of follow-up. The percentages above each curve indicate cumulative survival rates at 5 and 10 years. |
Of 16 variables evaluated, 3 were significantly related to recurrence on multivariate analyses: Child-Pugh class, type ofHCC, and regional cancer spread (table 4). The first 2 variableswere also significantly related to death. Early HCC was independentlyassociated with reduced risks of recurrence (relative risk, 0.31;95% CI, 0.15 to 0.65; P = .002) and death (relative risk, 0.26;95% CI, 0.09 to 0.73; P = .01) compared with overt HCC.
| View This table | table 4. Multivariate Analysis of Selected Variables on Endpoints in 67 Patients |
Discussion
This study shows that early HCC can be identified as a distinct clinical entity that has a high chance for surgical cure.In 15 patients with a single early HCC, the surgical outcome (5-yearoverall survival, 93%; recurrence-free survival, 47%) was encouragingas compared with that (54%; 16%) in 52 patients with overt HCC,as well as with the results of reported studies.15-19 Our durationof follow-up (median, 6.3 years) was long enough to confirm thisfinding; for 10 years and longer, a significant survival benefitin the early HCC group persisted. Minor hepatectomy with tumorclearance was well-tolerated (surgical mortality, 1.4%) and ensuredan optimal level of regional cure of early HCC (local recurrence,0%). Moreover, multivariate analysis showed that the early HCCgroup had a lower risk of death than did the overt HCC group (relativerisk, 0.26). These findings provide clinical evidence justifyingthe establishment of this new entity termed “earlyHCC.”
Questions arise with respect to the diagnosis of early HCC. What patients are likely to have early HCC? The mean diameterof tumors incidentally resected was 14 ± 4 mm (n = 33),4 suggestingthat patients with Stage I HCC are plausible candidates. In thisstudy, long-term monitoring (median, 5.4 years) mainly with ultrasonography(90%) of high-risk patients facilitated detection of small HCCsin endemic regions such as Japan, where the risk of HCC (yearlyincidence, 7%)20is higher than that in the West.21,22 Whyare early HCC difficult to diagnose? The insufficient diagnosticspecificity9 may be because the tumor itself does contain Glisson’striad and because it lacks distinct neoangiogenesis.1,4 Whatis the diagnostic approach to early HCC? To maximize chances foridentification, we used up to six imaging methods, including angiographicCT and Lipiodol-CT.10These additional methods resulted in fulfillmentof the diagnostic criteria in 25 patients (of whom 10 had earlyHCC). The risk of overdiagnosis (3 of 70 cases [4.3%]), however,was almost similar to that (21 of 590 cases [3.6%])23 reportedin a study of patients in whom HCC was diagnosed on the basisof currently accepted standards. The clinical diagnosis of earlyHCC therefore requires refined imaging criteria, such as ours.We cannot here propose the best diagnostic protocol; an additionof needle biopsy may simplify the process for future clinicalpractice. The final diagnosis in this series was made by the histologicalcriteria,4,5,13 which proved to discriminate early HCC bypresence or absence of atypia and invasion from precursor lesions.2
Recurrence of HCC remains the rule shortly after curative hepatic resection, with the rates ranging from 75% to 100% at 5 years.14-17,19 However, recurrence in the early HCC group generallyoccurred 3 years after surgery and was detected as a single nodulein a different segment in two thirds of the patients, suggestingmultifocal development of HCC.24 Multicentricity, however, isprobably not the sole cause of recurrence, because the cumulative5-year recurrence rate (53%) in this group was higher than therate of newly developed HCC (35%)20 in an at-risk cohort withcirrhosis alone. This difference may be explained by a study showingthat patients with a history of HCC are at greater risk of multicentrichepatocarcinogenesis than those without such a history.19Similarcoexistence rates of dysplastic lesions in surgical specimensmay indicate no remarkable difference between the two groups inthe risk of second primary HCC. It is possible that multifocaltumors or precursors (clinically occult at enrollment) may havebecome detectable at various times during postsurgical follow-up.22The surgical cure of early HCC, irrespective of the mode of oncogenesis,is likely to be evidenced by the absence of local recurrence,as well as by a long time (median, 3.9 years) to, and a reducedrisk (relative risk, 0.31) of,recurrence.
The 5-year survival after resection for early HCC (93%) contrasts sharply with reported results of Stage I HCC (46%-53%),19,25,26and is comparable with that after transplantation for incidentalor small HCC.27,28 Thus, a new subgrouping for this lesion,such as “Stage 0” HCC (not included in the current tumor-node-metastasisclassification),8 is of clinical value. The choice of therapyfor recurrence may have influenced the overall survival, but thedecision based on our criteria (not on pathological features ofthe initial tumor) was unlikely to be biased, because the differencebetween the two groups was not significant. Even when “local cure”of early HCC appears to have been achieved, over 90% survivalseems unlikely to last in these patients with cirrhosis that isassociated with “field cancerization.”14,16,24 In this respect,our outcome in the early HCC group shows a curability limit oflocal therapy that left the patients with a diseased liver, and,therefore, transplantation can be the only option for a potentially”absolutecure.”
Interpretation of our surgical outcome is probably affected by potential sources of lead time bias and length bias.29-31 Wehave already shown “less malignant” biological characteristicsof early HCC1,3 and a possible biological continuum fromprecursor via early to advanced HCC.2,4 Although informationon the natural history of early HCC is unavailable, the lesionitself may have a longer clinical course than overt HCC. The findingthat recurrence occurred later and less often in the early HCCgroup suggests that biology of the disease, rather than surgicalintervention for any specific lesion, plays an important rolein the difference in outcome. Taken together, early HCC may representan initial stage in the development of HCC, not just as concernsthe index lesion, but also with regard to the remaining liver.Ultimately, the only way to assess relevance of the biases wouldbe to clarify whether disease-specific mortality from HCC canbe reduced by screening or surveillance of at-risk subjects.31Even so, the promise of surgery for small HCCs suggests that earlydiagnosis and treatment for HCC seems justified, unless randomized,controlled trials recommend an alternativepolicy.
The concept of early HCC is based on a model of “stepwise” progression,2-4whereas an alternate hypothesis of de novo development32is now advocated; which theory applies better to our study populationremains to be elucidated. Further studies are needed to determinethe best diagnostic protocol, the screening-associated biases,and the optimal therapeutic option.33 Patients with early HCC were still a minority; the chance of detection, however, may have been underestimated, because only those who were deemed operable were recruited in this study. Our ongoing multicenter study has accumulated over 100 patients with early HCC, who underwent surgery or ethanol injection, to clarify the issues.
Early HCC is characterized not only by its incipient biological nature of cancer,1-4 but also by, as this study shows, an extremely favorable long-term outcome. We therefore conclude that our definition of early HCC can be justified clinicopathologically. Increased recognition of early HCC in routine clinical practice contributes to improved patients’ survival.
Acknowledgements
The authors thank Professor Emeritus Kunio Okuda, First Department of Medicine, Chiba University School of Medicine, for his critical review of the manuscript; Dr. Hidetaka Kawabata, Second Department of Surgery, Faculty of Medicine, University of Tokyo, for his helpful discussion on the data; and Dr. Kazuto Inoue, Department of Surgery, National Cancer Center Hospital East, and Dr. Chikuma Hamada, Department of Pharmacoepidemiology, Faculty of Medicine, University of Tokyo, for their help in statistical analysis.
Abbreviations: HCC, hepatocellular carcinoma; CT, computed tomography.
Footnotes
Supported by a grant-in-aid for Cancer Research and a grant-in-aid for the Comprehensive Ten-Year Strategy of Cancer Control from the Ministry of Health and Welfare of Japan.
Address reprint requests to: Tadatoshi Takayama, M.D., Ph.D., Second Department of Surgery, Faculty of Medicine, University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan. Fax: 81-3-5684-3989.
References
| 1. | Kanai T, Hirohashi S, Upton MP, Noguchi M, Kishi K, Makuuchi M, Yamasaki S, et al. Pathology of small hepatocellular carcinoma: a proposal for a new gross classification. Cancer 1987;60:810-819[Medline]. |
| 2. | Takayama T, Makuuchi M, Hirohashi S, Sakamoto M, Okazaki N, Takayasu K, Kosuge T, et al. Malignant transformation of adenomatous hyperplasia to hepatocellular carcinoma. Lancet 1990;336:1150-1153[Medline]. |
| 3. | Tsuda H, Zhang W, Shimosato Y, Yokota J, Terada M, Sugimura T, Miyamura T, et al. Allele loss on chromosome 16 associated with progression of human hepatocellular carcinoma. Proc Natl Acad Sci U S A 1990;87:6791-6794[Medline]. |
| 4. | Sakamoto M, Hirohashi S, Shimosato Y. Early stages of multistep hepatocarcinogenesis: adenomatous hyperplasia and early hepatocellular carcinoma. Hum Pathol 1991;22:172-178[Medline]. |
| 5. | International Working Party. Terminology of nodular hepatocellular lesions. HEPATOLOGY 1995;22:983-993 [ Link previously at www.ncbi.nlm.nih.gov ]. |
| 6. | Okuda K. Early recognition of hepatocellular carcinoma. HEPATOLOGY1986;6:729-738[Medline]. |
| 7. | Takayama T, Makuuchi M, James ND, Kerr D. Hepatocellular carcinoma. In: McCulloch P, Kingsnorth A (eds). Management of Gastrointestinal Cancer. London: BMJ Publishing Group, 1996:283-299. |
| 8. | International Union Against Cancer. Liver. In: Sobin LH, Wittekind CH (eds). TNM Classification of Malignant Tumours. 5th ed. New York: Wiley-Liss Inc., 1997:74-77. |
| 9. | Takayasu K, Furukawa H, Wakao F, Muramatsu Y, Abe H, Terauchi T, Winter TC III, et al. CT diagnosis of early hepatocellular carcinoma: sensitivity, findings, and CT-pathologic correlation. Am J Roentgenol 1995;164:885-890. |
| 10. | Takayasu K, Muramatsu Y, Furukawa H, Wakao F, Moriyama N, Takayama T, Yamasaki S, et al. Early hepatocellular carcinoma: appearance at CT during arterial portography and CT arteriography with pathologic correlation. Radiology 1995;194:101-105 [ Link previously at www.ncbi.nlm.nih.gov ]. |
| 11. | Makuuchi M, Kosuge T, Takayama T, Yamazaki S, Kakazu T, Miyagawa S, Kawasaki S. Surgery for small liver cancers. Semin Surg Oncol 1993;9:298-304 [ Link previously at www.ncbi.nlm.nih.gov ]. |
| 12. | Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer 1954;7:462-503. |
| 13. | Kojiro M, Sugihara S, Nakashima O. Pathomorphologic characteristics of early hepatocellular carcinoma. In: Okuda K, Tobe T, Kitagawa T (eds). Early Detection and Treatment of Liver Cancer. Tokyo: Japan Scientific Societies Press, 1991:29-37. |
| 14. | Yamamoto J, Kosuge T, Takayama T, Shimada K, Yamasaki S, Ozaki H, Yamaguchi N, et al. Recurrence of hepatocellular carcinoma after surgery. Br J Surg 1996;83:1219-1222 [ Link previously at www.ncbi.nlm.nih.gov ]. |
| 15. | Belghiti J, Panis Y, Farges O, Benhamou JP, Fekete F. Intrahepatic recurrence after resection of hepatocellular carcinoma complicating cirrhosis. Ann Surg 1991;214:114-117 [ Link previously at www.ncbi.nlm.nih.gov ]. |
| 16. | Sasaki Y, Imaoka S, Masutani S, Ohashi I, Ishikawa O, Koyama H, Iwanaga T. Influence of coexisting cirrhosis on long-term prognosis after surgery in patients with hepatocellular carcinoma. Surgery 1992;112:515-521 [ Link previously at www.ncbi.nlm.nih.gov ]. |
| 17. | Kosuge T, Makuuchi M, Takayama T, Yamamoto J, Shimada K, Yamasaki S. Long-term results after resection of hepatocellular carcinoma: experience of 480 cases. Hepatogastroenterology 1993;40:328-332 [ Link previously at www.ncbi.nlm.nih.gov ]. |
| 18. | Nagasue N, Kohno H, Chang YC, Taniura H, Yamanoi A, Uchida M, Kimoto T, et al. Liver resection for hepatocellular carcinoma: results of 229 consecutive patients during 11 years. Ann Surg 1993;217:375-384 [ Link previously at www.ncbi.nlm.nih.gov ]. |
| 19. | Kumada T, Nakano S, Takeda I, Sugiyama K, Osada T, Kiriyama S, Sone Y, et al. Patterns of recurrence after initial treatment in patients with small hepatocellular carcinoma. HEPATOLOGY 1997;25:87-92 |
| 20. | Oka H, Kurioka N, Kim K, Kanno T, Kuroki T, Mizoguchi Y, Kobayashi K. Prospective study of early detection of hepatocellular carcinoma in patients with cirrhosis. HEPATOLOGY 1990;12:680-687 |
| 21. | Omata M. Current perspectives on hepatocellular carcinoma in Oriental and African countries compared to developed Western countries. Dig Dis 1987;5:97-115 [ Link previously at www.ncbi.nlm.nih.gov ]. |
| 22. | Colombo M, de Franchis R, Del Ninno E, Sangiovanni A, De Fazio C, Tommasini M, Donato MF, et al. Hepatocellular carcinoma in Italian patients with cirrhosis. N Engl J Med 1991;325:675-680 [ Link previously at www.ncbi.nlm.nih.gov ]. |
| 23. | Shimizu S, Takayama T, Kosuge T, Yamamoto J, Shimada K, Yamazaki S, Hasegawa H, et al. Benign tumors of the liver resected because of a diagnosis of malignancy. Surg Gynecol Obstet 1992;174:403-407 [ Link previously at www.ncbi.nlm.nih.gov ]. |
| 24. | Muto Y, Moriwaki H, Ninomiya M, Adachi S, Saito A, Takasaki KT, Tanaka T, et al. Prevention of second primary tumors by an acyclic retinoid, polyprenoic acid, in patients with hepatocellular carcinoma. N Engl J Med 1996;334:1561-1567. |
| 25. | The Liver Cancer Study Group of Japan. Predictive factors for long term prognosis after partial hepatectomy for patients with hepatocellular carcinoma in Japan. Cancer 1994;74:2772-2780 [ Link previously at www.ncbi.nlm.nih.gov ]. |
| 26. | Nagashima I, Hamada C, Naruse K, Osada T, Nagao T, Kawano N, Muto T. Surgical resection for small hepatocellular carcinoma. Surgery 1996;119:40-45 [ Link previously at www.ncbi.nlm.nih.gov ]. |
| 27. | Iwatsuki S, Gordon RD, Shaw BW Jr, Starzl TE. Role of liver transplantation in cancer therapy. Ann Surg 1985;202:401-407[Medline]. |
| 28. | Bismuth H, Chiche L, Adam R, Castaing D, Diamond T, Dennison A. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann Surg 1993;218:145-151 [ Link previously at www.ncbi.nlm.nih.gov ]. |
| 29. | Dusheiko GM, Hobbs KEF, Dick R, Burroughs AK. Treatment of small hepatocellular carcinomas. Lancet 1992;340:285-288 [ Link previously at www.ncbi.nlm.nih.gov ]. |
| 30. | Black WC, Welch HG. Screening for disease. Am J Roentgenol 1997;168:3-11. |
| 31. | Collier J, Sherman M. Screening for hepatocellular carcinoma. HEPATOLOGY 1998;27:273-278 |
| 32. | Theise ND, Marcelin K, Goldfischer M, Hytiroglou P, Ferrell L, Thung SN. Low proliferative activity in macroregenerative nodules: evidence for an alternate hypothesis concerning human hepatocarcinogenesis. Liver 1996;16:134-139 [ Link previously at www.ncbi.nlm.nih.gov ]. |
| 33. | Castells A, Bruix J, Bru C, Fuster J, Vilana R, Navasa M, Ayuso C, et al. Treatment of small hepatocellular carcinoma in cirrhotic patients: a cohort study comparing surgical resection and percutaneous ethanol injection. HEPATOLOGY 1993;18:1121-1126 |