Original Articles Evaluation of Assays for Antibody to Hepatitis E Virus by a Serum Panel
|
| table 3. Antibody to HEV Endpoint Dilution by 12 Different Tests in Early-Convalescent Human Sera Diluted With Normal Human Serum |
Seven of the eight recombinant protein tests detected anti-HEV in 5 convalescent human sera obtained 2 months to 13 years following onset of acute Hepatitis E (data not shown). One recombinant protein test detected anti-HEV in sera obtained 2 months to 2 years after illness onset, but was indeterminate in serum obtained 13 years after illness onset. Only one of the four synthetic peptide tests detected anti-HEV in all four sera obtained 2 months to 2 years after illness onset, and none of the synthetic peptide tests detected anti-HEV in serum obtained 13 years after illness onset.
Six tests detected anti-HEV in more than 85% of serial chimpanzee sera after inoculation with HEV strains from various geographic regions (table 4). However, there was considerable variation in the pattern of anti-HEV detection by these assays, in sera collected both <6 months and ![]() 6 months after inoculation. Tests that included ORF3 epitopes from the Mexican HEV strain (tests 6, 7, 8, 11, and 12) did not detect anti-HEV in several chimpanzee sera after inoculation with this HEV strain (table 4).
6 months after inoculation. Tests that included ORF3 epitopes from the Mexican HEV strain (tests 6, 7, 8, 11, and 12) did not detect anti-HEV in several chimpanzee sera after inoculation with this HEV strain (table 4).
| table 4. Detection of Anti-HEV by 12 Different Tests in Sera From Chimpanzees Inoculated With HEV Strains From Various Geographic Regions |
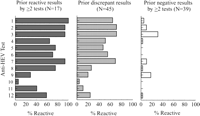
The detection of anti-HEV in blood donor sera varied considerably among assays. The range of anti-HEV detection in 17 sera that were previously reactive by at least two tests was 6% to 100%, the range of detection in 45 sera with prior discordant results was 4% to 71%, and the range of detection in 39 sera with prior negative results by at least two tests was 0% to 31% (Fig. 1). In pairwise comparisons of different tests, the overall concordance in all blood donor sera ranged from 41% to 94% (median, 68%) and the concordance among reactive sera by either test ranged from 0% to 89% (median, 32%) (table 5). Only 23% (15/66) of the pairwise test comparisons had concordant results for >50% of reactive sera.
|
|
Fig. 1. Antibody to HEV reactivity in blood donor sera with prior reactive results by |
| table 5. Pairwise Test Comparisons of Concordant Detection of Antibody to HEV among U.S. Blood Donor Sera |
DISCUSSION
Most currently available assays for anti-HEV were designed for the diagnosis of acute HEV infection acquired in HEV-endemic regions. The findings of this study indicate that several of the recombinant protein assays (tests 1, 2, 4, 5, and 7) have an adequate combination of sensitivity and specificity to perform well for this purpose (table 6). The peptide-based assays were generally much less sensitive compared to the recombinant protein assays and are therefore likely to be nonreactive in a high proportion of acute Hepatitis E cases. Further comparative studies that include testing for immunoglobulin M anti-HEV would be useful to validate the performance of the recombinant protein assays for the diagnosis of acute Hepatitis E. In addition, the performance of these assays for the diagnosis of acute Hepatitis E in persons who do not have a history of travel to HEV-endemic regions needs to be determined.
| table 6. Overall Performance of 12 Tests for Antibody to HEV |
In prior studies, HEV isolates from various geographic regions have been demonstrated to have at least one major cross-reactive epitope by a variety of serologic assays. 5,10,32,33 However, we found substantial variation in the detection of anti-HEV by these tests in acute and early convalescent-phase sera from chimpanzees infected with HEV isolates from various geographic regions. One possible reason for these findings is differences in the geographic strain-specific antigenic domains included in these tests. However, there is little variation in the RNA sequence of ORF2 among HEV isolates from various geographic regions. Moreover, some assays (tests 6, 7, 8, and 12) did not detect anti-HEV in chimpanzee sera even though these tests included ORF3 epitopes from the same geographic region as the chimpanzee inoculum. The seroreactivity of recombinant proteins may also vary if they are produced in different expression systems or used in different test formats (i.e., Western blot vs. EIA). In addition, all of these assays were designed to detect human antibody, and differences may exist in the ability of assay conjugates to detect chimpanzee antibody. However, if the assay conjugate were the reason for a test’s not detecting anti-HEV, the assay would be expected either to be nonreactive in all the chimpanzee sera or to have a uniform decline in seroreactivity in chimpanzee sera compared to human sera. None of the assays that consistently detected anti-HEV in human sera exhibited either of these patterns of seroreactivity in chimpanzee sera.
Anti-HEV assays used in seroprevalence studies must have a high level of sensitivity in detecting remote infection. Several tests detected anti-HEV in >90% of chimpanzee and human sera obtained 6 months to 2 years after infection, and all but one of the recombinant protein assays detected anti-HEV in a human serum obtained 13 years after infection. However, our ability to assess the sensitivity of these assays in detecting remote infection was limited because only a small number of such sera were included in the panel. Further studies are needed to determine the ability of these tests to detect anti-HEV in patients with remote infection.
Anti-HEV tests used in seroprevalence studies must have a high level of specificity; this is particularly important to prevent false-positive tests in populations with a low prevalence of infection. Most of these assays did not detect anti-HEV in any of the 22 known-negative sera in the panel. However, among the 101 selected blood donor sera included in the panel, anti-HEV seroreactivity was highly variable, and the concordance in detection of anti-HEV between tests was low. Possible reasons for these findings include nonspecific reactivity and differences in the sensitivity of these assays in detecting antibodies to different HEV strains and in detecting remote infection.
Seroprevalence studies among blood donors in some non-HEV-endemic countries have found an anti-HEV prevalence of 1% to 20%, which is relatively high compared to the low rate of clinically evident disease associated with HEV in these areas. 7,24-27 In one study, anti-HEV seroreactivity among persons living in nonendemic regions increased with increasing age and was associated with travel to endemic regions, findings that are consistent with prior HEV infection.24 However, in another study, there was no evidence that anti-HEV seroreactivity was related to subclinical infection in high risk populations for Hepatitis A virus, Hepatitis B virus, and Hepatitis C virus infections.27 Thus, the interpretation of seroreactivity among persons living in nonendemic regions is currently problematic. Moreover, the discrepant results among blood donor sera in this study indicate that anti-HEV seroprevalence data in nonendemic countries may be unreliable and should be interpreted with caution. Further studies are needed to determine reasons for the highly discrepant results among blood donor sera. Studies are also needed to determine the significance of anti-HEV seroreactivity among persons living in non-HEV-endemic areas, including the relation of seroreactivity to exposure to the recently discovered virus in pigs that is closely related to human HEV isolates.34 In addition, improved tests are needed for use in seroprevalence studies in nonendemic regions and confirmation tests are needed to verify the specificity of these assays.
Footnotes
Acknowledgement: The authors thank Stephen Lambert, Karen McCaustland, John Spelbring, and Doris Wong for assistance in constructing the coded serum panel.
Abbreviations: anti-HEV, antibody to Hepatitis E virus; HEV, Hepatitis E virus; EIA, enzyme immunoassay; ORF, open reading frame.
The Hepatitis E Virus Antibody Serum Panel Evaluation Group included H. J. Alter, National Institutes of Health, Bethesda, MD; D. Anderson, McFarlane Burnet Centre for Medical Research, Fairfield, Victoria, Australia; M. S. Balayan, Institute of Poliomyelitis and Viral Encephalitis, Moscow, Russia; A. N. Burkov, NPO-Diagnostic Systems, Nizhny Novgorod, Russia; L. Chan, Genelabs Diagnostics, Singapore; P. Coursaget, Laboratoire d’Immunologie des Maladies Infectieuses, Tours, France; M. O. Favorov, Centers for Disease Control and Prevention, Atlanta, GA; K. Krawczynski, Centers for Disease Control and Prevention, Atlanta, GA; I. K. Mushahwar, Abbott Laboratories, North Chicago, IL; S. K. Panda, All India Institute of Medical Sciences, New Delhi, India; J. Pillot, Institute Pasteur, Paris, France; S. A. Tsarev, National Institutes of Health, Bethesda, MD; T. Uchida, Nihon University School of Medicine, Tokyo, Japan; and P. O. Yarbough, Genelabs Technologies, Inc., Redwood City, CA.
Received October 28, 1996; accepted November 5, 1997.
Address reprint requests to: Eric E. Mast, M.D., M.P.H., Hepatitis Branch, Mailstop G37, Centers for Disease Control and Prevention, 1600 Clifton Road, Atlanta, GA 30333. Fax: (404) 639-1538.
Copyright © 1998 by the American Association for the Study of Liver Diseases.