Hepatology, December 1998, p. 1724-1724, Vol. 28, No. 6
Correspondence
Diurnal Variation of Serum Alanine Transaminase Activity in Chronic Liver Disease
The determination of serum levels of alanine transaminase (ALT) is a critical tool in the diagnosis and follow up of patients with liver disease. We were interested in the recent article by Piton et al.,1 which identified factors associated with ALT variability from a cohort of 1,033 blood donors and estimated the clinical impact of correcting the normal upper limit value of ALT according to these parameters. However, the authors did not mention if blood sampling was obtained at the same time of the day and if they assessed the role of this factor on ALT values. In healthy individuals there is a such a variation of ALT2 and other blood analytes3of low amplitude that does not posediagnostic problems. However, in patients with high values ofALT, the changes could be of significance. Different biologicalrhythms have been shown to be abnormal in patients with cirrhosis4-6and in rats with portacaval anastomosis.7,8 It is unknown whether a common abnormality may also affect the diurnal variation of ALT.
We examined the diurnal variation of serum ALT in a group of 12 patients with cirrhosis (5 men, 7 women; 35-63 years; meanage, 47 years) of different etiologies (5 alcoholic, 4 hepatitis C, and 3 primary biliary cirrhosis) staged Child-Pugh class A in 4 subjects and Child-Pugh class B in 8 subjects. All were medically stable and followed a standardized protocol4 as part of a study to examine circadian rhythms in cirrhosis. Patients were submitted to hourly venous sampling. In each patient, ALT values were simultaneouslyassessed in all 24 samples using a standard automatic analyzer(Beckman Synchron CX-7, Beck, CA). The intra-assay coefficientof variation (5%) was calculated comparing duplicates of 120 samples (hourly samples of 5 patients).
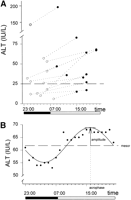
The median of the mean values of hourly determinations of ALT was 34 IU/L (range, 6-195 IU/L). The range of change ([highestvalue ![]() lowest value]/lowest value) was 45% (range, 18-54%). In 3 patients the values of ALT changed from below to above the upper limit of normality (Fig. 1A). Serial measurement of serum levels of liver enzymes disclosed higher levels during the day (8 AM-10PM; median, 38 IU/L; first quartile, 28 IU/L) than during the night (11 PM-7 AM; median, 33 IU/L; first quartile, 26 IU/L; paired Student’s t test P = .023). Evaluation of data by the population-mean cosinor method9 (fitting the curve to the mean values of the population at each data point) showed a statistically significant circadian variation (rhythm detection P < .001) with peak values for liver enzymes (acrophase) localized in the afternoon (15:37). Individual models (fitting the curve to the mean values of each patient at each data point; Fig. 1B) still kept a high goodnessof fit (R2 = 44 ± 17) with a considerable range of change (2 × amplitude = 22 ± 6%).
lowest value]/lowest value) was 45% (range, 18-54%). In 3 patients the values of ALT changed from below to above the upper limit of normality (Fig. 1A). Serial measurement of serum levels of liver enzymes disclosed higher levels during the day (8 AM-10PM; median, 38 IU/L; first quartile, 28 IU/L) than during the night (11 PM-7 AM; median, 33 IU/L; first quartile, 26 IU/L; paired Student’s t test P = .023). Evaluation of data by the population-mean cosinor method9 (fitting the curve to the mean values of the population at each data point) showed a statistically significant circadian variation (rhythm detection P < .001) with peak values for liver enzymes (acrophase) localized in the afternoon (15:37). Individual models (fitting the curve to the mean values of each patient at each data point; Fig. 1B) still kept a high goodnessof fit (R2 = 44 ± 17) with a considerable range of change (2 × amplitude = 22 ± 6%).
In summary, we observed higher values of liver enzymes during the day than at night and a peak time in the afternoon. Thus, serum values of liver enzymes need to be interpreted with consideration of their diurnal variation. Piton et al.1 reported variations in the percentage of individuals that would be classified as having normal ALT that ranged from 11% to 17% depending on the group examined (blood donors, hepatitis C patients, or responders to interferon). According to our results variations of similar range could be present comparing samples obtained at different times of the day. This could be relevant because in many centers inpatients are submitted to blood sampling in the morning whereas outpatients are submitted to blood sampling in theafternoon.
We observed that the diurnal rhythm of ALT in cirrhosis is preserved and similar to the diurnal rhythm described in healthyindividuals.2,3 Furthermore, the rhythm is constant as shown in 5 patients that had the study repeated 1 month after the first admission and had no significant changes in rhythm parameters (Spearman’s correlation coefficient for mesor = 0.8 and for acrophase = 1). The mechanism that regulates the diurnal variation of blood analytes is unknown but, according to these data, does not appear to be affected by cirrhosis.
In conclusion, as pointed out by Piton et al., several factors need to be considered when using liver enzymes in the clinical assessment of liver disease. Among them, circadian variation may be especially important when enzyme values are compared in a patient with chronic liver disease that had blood sampled at different times of theday.
| Juan Córdoba, M.D. Ken O’Riordan, M.D. Josée Dupuis, Ph.D. Jayme Borensztajn, M.D. Andres T. Blei, M.D. Departments of Medicine, Preventive Medicine, and Pathology Lakeside Veterans Affairs Medical Center and Northwestern University Chicago, IL |
| 1. | Piton A, Poynard T, Imbert-Bismut F, Khalil L, Delattre J, Pelissier E, Sansonetti N, et al. Factors associated with serum alanine transaminase activity in healthy subjects: consequences for the definitions of normal values, for selection of blood donors, and for patients with chronic hepatitis C. HEPATOLOGY 1998;27:1213-1219 [Abstract/Full Text] |
| 2. | Rivera-Coll A, Fuentes-Arderiu X, Diez-Noguera A. Circadian rhythms of serum concentrations of 12 enzymes of clinical interest. Chronobiol Int 1993;10:190-200 [Medline] |
| 3. | Haus E, Touitou Y. Chronobiology in laboratory medicine. In: Touitou Y, Haus E (eds). Biologic Rhythms in Clinical and Laboratory Medicine. Berlin: Springer, 1992:674-708. |
| 4. | Moller S, Winberg N, Henriksen JH. Non-invasive 24-hour ambulatory arterial blood pressure monitoring in cirrhosis. HEPATOLOGY 1995;22:88-95[Abstract]. |
| 5. | Steindl PE, Finn B, Bendok B, Rothke S, Zee PC, Blei AT. Disruption of the diurnal rhythm of plasma melatonin in cirrhosis. Ann Intern Med 1995;123:274-277 [Medline] |
| 6. | Córdoba J, Cabrera J, Lataif L, Penev P, Zee P, Blei AT. High prevalence of sleep disturbance in cirrhosis. HEPATOLOGY 1998;27:339-345[Full Text] |
| 7. | Zee PC, Mehta R, Turek FW, Blei AT. Portacaval anastomosis disrupts circadian locomotor activity and pineal melatonin rhythms in rats. Brain Res 1991;560:17-22 [Medline] |
| 8. | Córdoba J, Gottstein J, Blei AT. Stenosis of a portacaval anastomosis affects circadian locomotor activity: a multivariable analysis. Am J Physiol 1997;273 (Gastrointest Liver Physiol 36):G1218-G1225 [Medline] |
| 9. | Monk TH. Research methods of chronobiology. In: Webb WB (ed). Biological Rhythms, Sleep and Performance. New York: John Wiley & Sons, 1982:27-57. |